-
PDF
- Split View
-
Views
-
Cite
Cite
Lara Wirth, Kevin Spencer, Larry Benge, George Dimitroulis, Custom subperiosteal implants used to rehabilitate the atrophic edentulous maxilla following multiple failures of both conventional as well as zygomatic implants: a case report, Journal of Surgical Case Reports, Volume 2025, Issue 10, October 2025, rjaf319, https://doi.org/10.1093/jscr/rjaf319
Close - Share Icon Share
Abstract
The treatment of cleft lip and cleft palate patients typically involves numerous surgeries, often including the replacement of missing teeth and endosseous dental implants are often utilized in this context. Endosseous dental implants require adequate bone volume for placement, and in this patient cohort this often requires an initial alveolar bone graft. Technological advancements can now harness computer-aided design and computer-aided manufacturing (CAD-CAM) to produce modern custom subperiosteal implants that utilize the available bone surface, thus providing an alternative treatment for patients unable to have conventional dental implants. We report the case of a 72-year-old male cleft palate patient who failed a combination of both regular endosseous and zygomatic implants and was left with both hard and soft tissue defects, such that he was not a candidate for further conventional implant placement. Using CAD-CAM technology, custom subperiosteal implants were created to successfully treat his atrophic and severely deformed edentulous maxilla.
Introduction
Cleft lip and cleft palate (CP) are the most common congenital orofacial deformities affecting 1 in every 1000–1500 births globally [1]. Treatment of these patients involves multiple staged surgeries from childhood to young adulthood, including but not limited to; cheilorhinoplasty, palatoplasty, orthognathic surgery, removal of malformed and supernumerary teeth, alveolar grafting and endosseous implant placement [2]. A high incidence of poor dental hygiene in CP patients can exacerbate tooth loss, and in severe cases this can even lead to complete edentulousness [3].
Zygomatic implants (ZIs) are a well-documented standard treatment for the severely atrophic maxilla [4]. An additional solution for patients with atrophic edentulous jaws utilizes technological advancements in computer-aided design and computer-aided manufacturing (CAD-CAM) tools to create patient-specific subperiosteal implants (SIs) [5]. A patients' CT scan is used to digitally design the custom implant for hermetic approximation with the native bone surface. After approval from the oral and maxillofacial surgeon and prosthodontist/restorative dentist, the implant is 3D printed in medical-grade titanium. One such implant approved for use in Australia is the Osseoframe™. The following report illustrates the novel use of SIs in conjunction with conventional implants in a CP case where both the conventional implants as well as ZIs failed.
Case report
A 72-year-old male patient first presented to the clinic ⁓10 years prior with an unrepaired, complete, unilateral CP. The patient’s medical history was unremarkable apart from a history of heavy smoking along with terminal maxillary and mandibular dentition making him a poor candidate for alveolar bone grafting.
Over the next decade he underwent multiple implant procedures including the placement of ZIs and standard dental implants which subsequently had to be removed due to complications including; infection, chronic sinusitis, bone loss at implantation sites, and integration failure.
As a result of these previous surgeries, the patient was edentulous with an atrophic maxilla, complicated by a large CP defect. All that remained from his previous surgeries were the maxillary tuberosity implants. Given the lack of available hard and soft tissue due to the patient’s complex maxillary deformity, further implantation was not possible.
It was decided that in order to rehabilitate this complex case, two custom titanium SIs would be utilized in combination with the two remaining tuberosity implants in order to stabilize an implant retained obturator (Figs 1 and 2).
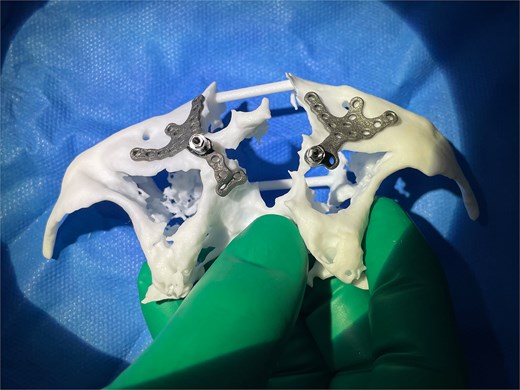
Left and right custom Osseoframes™ positioned on a 3D printed model of patient's native bone prior to implantation.
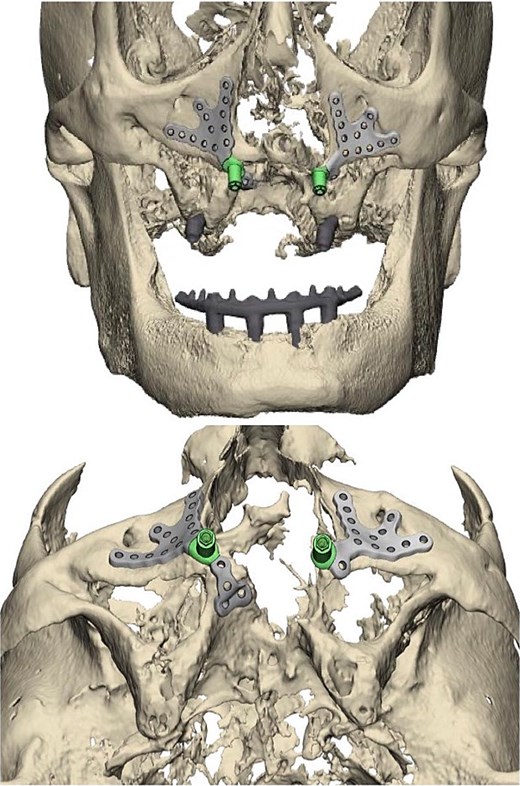
Left and right custom subperiosteal implant placement digitally modeled in pre-surgical report provided by product manufacturer.
Under general anesthesia, the anterior maxilla was accessed by raising a full-thickness mucoperiosteal flap. The two SIs were placed in situ and secured with multiple 1.6 mm self-tapping screws placed onto the available buccal and palatal bone (Fig. 3). On top of each transmucosal abutment was attached a 17°, 3 mm zygoma multiunit with standard multiunit cap. Zest abutments were left on the tuberosity implants. The subperiosteal frames were covered with mucoperiosteal flaps and the surgery was completed uneventfully.
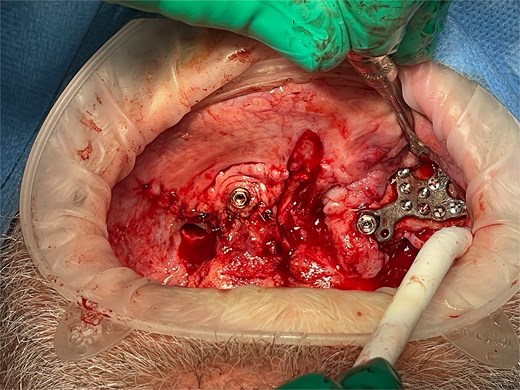
Intraoperative photo showing the right subperiosteal post emerging through the gingiva after soft tissue closure. The left subperiosteal implant frame and post is exposed with soft tissue retracted.
Digital scans were taken at 3 months to allow for the construction of the metal framework. After try-in and adjustment appointments, the prosthesis was inserted 6 months post-surgery (Fig. 4) and the patient was successfully rehabilitated (Fig. 5). At the time of reporting there have been no post-surgical complications.
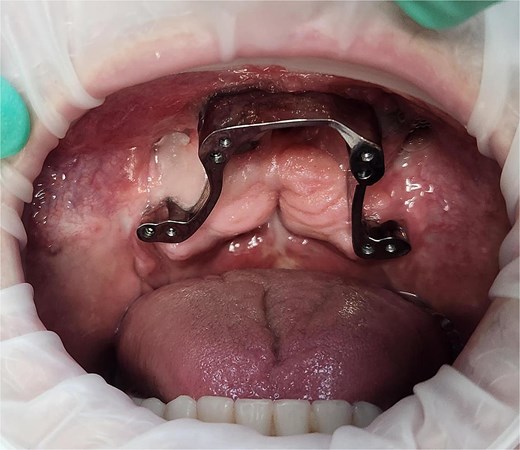
Photograph of the dental bar used to support the final dental prosthesis 6 months following surgery. Note the pre-existing tuberosity implants posteriorly.
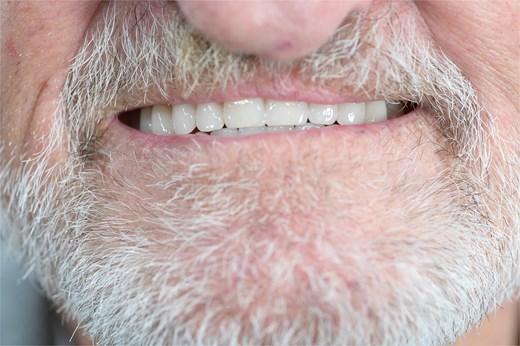
Photograph of the patient's prosthesis supplied by the combination of subperiosteal and endosseous implants 6-months post-surgery.
Discussion
Developmental dental abnormalities are a well-documented issue affecting CP patients. Currently, dental implants are considered the best option for restoration of missing teeth [6]. However, their success depends on the quality and quantity of residual bone, often necessitating bone augmentation [7]. ZIs provide an alternative to conventional dental implants and bone grafting for patients with atrophic maxillae.
In cases where either conventional implants or ZIs fail, SIs offer a novel solution for complex edentulous patients. Unlike conventional implants or ZIs, SIs created using CAD-CAM technology are personalized, utilizing the patients’ anatomy to provide a bespoke solution. The less invasive nature of SIs allows for a relatively short, single-staged operation with lower morbidity than traditional implants [8].
Stress modeling using finite element analysis has demonstrated that the functional load of the SI is distributed across the area of bone covered by the custom baseplate frame [5, 9]. Stability of SIs confers the important advantage of allowing for immediate functional loading with a custom dental prosthesis at time of surgery [9]. Enhanced stability also offers the possibility to treat large maxillary defects, providing an option for patients unwilling or unsuitable to undergo other more complex procedures [10].
The most documented complication of SIs is gingival dehiscence resulting in partial exposure of framework at the base of the transmucosal posts, and in select cases this leads to implant failure. A study by Dimitroulis et al. on 21 cases treated with Osseoframes™ reported five instances of framework exposure, three of which required replacement with new implant frames [5]. Several studies have reported that exposure does not impact patient’s comfort, functioning, prosthesis stability, or patient satisfaction [11]. Advancements in design and surgical techniques continue to reduce the incidence of SI complications [5, 11].
Due to the personalized nature of CAD-CAM implant production, it takes 2–4 weeks to design, produce and deliver a custom SI. As technology improves, production times are expected to decrease, increasing accessibility and improving affordability. Lack of data from long-term clinical studies currently limits understanding of SI durability and effectiveness. As demonstrated with this case, SIs were able to be used in a novel way to resurrect a complex case that had failed all other treatment modalities.
Acknowledgements
The contribution of MAXONIQ (www.maxoniq.com) who design, develop, and manufacture the subperiosteal implant device, commercially known as the Osseoframe™, is gratefully acknowledged.
The contribution of Next Smile Australia who provided patient treatment and supplied information for this report, is gratefully acknowledged.
Declaration
GD is the Founder and Clinical Director of MAXONIQ, the company that developed and supplied the subperiosteal implants described in this report.
The patient provided informed consent for this case report to be written.
Conflict of interest statement
None declared.
Funding
None declared.
References
- cleft palate
- computer-aided design
- computers
- endosseous dental implantation
- dental implants
- maxilla
- mouth, edentulous
- surgical procedures, operative
- tooth loss
- tissue transplants
- atrophic condition of skin
- cleft palate with cleft lip
- implants
- soft tissue
- alveolar bone of maxilla
- printing, three-dimensional
- atrophic maxilla



