-
PDF
- Split View
-
Views
-
Cite
Cite
Diana Nouh, Maisoon Dayoub, Moatasem Hussein Al-janabi, Mucinous cystadenoma of the ovary with xanthogranulomatous oophoritis: the first case report in the literature, Journal of Surgical Case Reports, Volume 2024, Issue 3, March 2024, rjae155, https://doi.org/10.1093/jscr/rjae155
Close - Share Icon Share
Abstract
This case report presents a unique and unprecedented occurrence of mucinous cystadenoma of the ovary accompanied by xanthogranulomatous oophoritis, a rare inflammatory condition. To the best of our knowledge, this is the first documented case of its kind in the medical literature. The patient, a 25-year-old woman, presented with abdominal pain, fever, and discomfort, prompting further investigation that led to the unexpected discovery of these coexisting pathologies.
Introduction
Xanthogranulomatous inflammation is an uncommon form of chronic inflammation characterized by the replacement of normal tissues of the affected organ by foamy macrophages with an admixture of lymphocytes, plasma cells, and neutrophils, causing a functional failure of the affected organ [1]. The most commonly affected organs are the kidney and gallbladder, followed by anorectal area, bone, stomach, and testis [2]. If the inflammation occurs in the female genital tract, it more commonly affects the endometrium, but the vagina, cervix, fallopian tube, and ovary are very rare [2]. The etiology of xanthogranulomatous oophoritis is unknown, but it shares histopathological findings similar to those of xanthogranulomatous change occurring in various organs, including the gallbladder and kidney [2]. On the other hand, ovarian mucinous cystadenoma is a benign tumor that arises from the surface epithelium of the ovary. It is a multilocular cyst with smooth outer and inner surfaces that occurs mainly during the third to sixth decades, but it may also occur in younger women [3, 4]. About 80% of ovarian mucinous tumors are benign [4]. Although each of these entities has been reported independently in the literature, the coexistence of mucinous cystadenoma and xanthogranulomatous oophoritis within the same ovarian tissue has not been previously documented.
Case presentation
A 25-year-old woman, nulliparous, presented to our gynecology clinic with complaints of intermittent low-grade fever with lower abdominal pain, most severe in the left iliac fossa, and distension over the last 2 months. These symptoms were not associated with any gastrointestinal or urinary complaints. The patient had a history of a left ovarian cyst and underwent a laparoscopy 4 months ago, initially relieving her symptoms. However, after 2 months, the symptoms recurred, accompanied by a high fever with a high C-reactive protein (CRP) level. Due to the inflammatory symptoms and the ultrasound findings, the condition was diagnosed as an ovarian abscess, and a course of antibiotics was administered. However, the patient did not show improvement, and a persistent low-grade fever accompanied by consistently elevated CRP levels continues to be observed. Her menstrual history was normal with regular periods. There was no family history of breast, ovarian, or endometrial cancer. On physical examination, the abdomen was tender, and a palpable mass was felt in the left iliac fossa region. Pelvic examination revealed a normal-sized non-pregnant firm uterus and fullness in the cul-de-sac and left adnexa. Subsequent investigations showed hemoglobin (Hb) of 10.9 g/dl, total leucocytes count of 15.7 x 10 g/l, erythrocyte sedimentation rate of 56 mm/h, CRP level of 24.6 mg/l (normal range 0–6 mg/l), and CA-125 (cancer antigen 125) of 12.5 U/ml (normal range 0–20.1 U/ml). Ultrasound of the abdomen and transvaginal ultrasound were performed revealing a 9 cm x 6 cm thick-walled cystic mass in the left pelvic region. The mass exhibits irregular borders, a heterogeneous echotexture, and is multilocular with numerous septations (Fig. 1). The right ovary was not visualized separately. The patient underwent a unilateral salpingo-oophorectomy, and intraoperative findings confirmed a large cystic mass with a nodular appearance arising from the left ovary (Fig. 2) (Video 1). The right ovary appeared grossly normal and was preserved to maintain hormonal function. On gross examination, the surgically excised specimen consisted of a cystic mass measuring 9 cm x 7 cm x 5 cm, exhibiting nodules. Upon sectioning, the mass had a thick outer wall and was multilocular with variably sized septa, containing thick gelatinous mucoid material characteristic of a mucinous tumor. No solid areas or papillary structures were noted. Surprisingly, the adjacent tissue displayed yellowish nodules and areas of firm induration (Fig. 3). Microscopic examination of the mass revealed cystic glands lined by a single layer of non-ciliated columnar epithelium with abundant intracellular mucin. No cytologic atypia or mitotic figures. Papillary components and stromal invasion were not identified. Subepithelial-marked foamy macrophages, fibrosis, and chronic inflammatory infiltrates were observed (Fig. 4). Based on histopathological features, a diagnosis of mucinous cystadenoma with xanthogranulomatous oophoritis was made. Postoperatively, the patient’s recovery was uneventful, and follow-up assessments at 6 months revealed no evidence of recurrence.
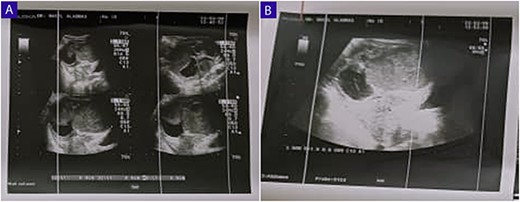
(A and B) Ultrasound of the abdomen and TVUS reveals a 9 cm x 6 cm thick-walled cystic mass in the left pelvic region; the mass exhibits irregular borders, a heterogeneous echotexture, and is multilocular with numerous septations.
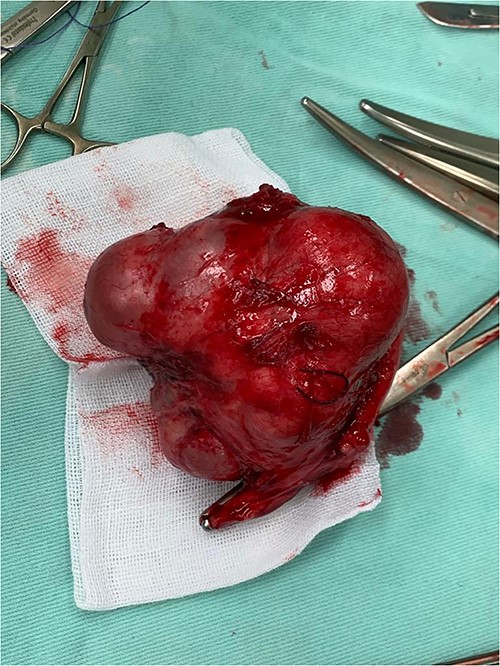
A surgical image shows a large cystic mass with a nodular appearance arising from the left ovary.
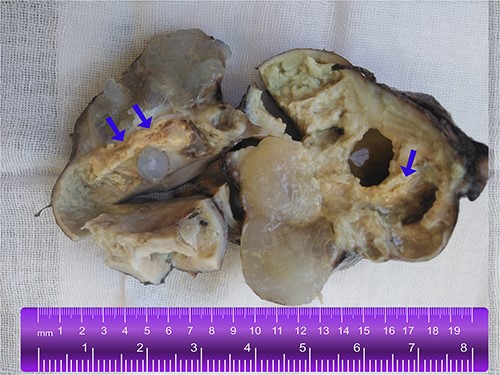
A gross image of the specimen shows a cystic mass measuring 9 cm x 7 cm x 5 cm; upon sectioning, the cyst is multilocular with variably sized septa, containing thick gelatinous mucoid material characteristic of a mucinous tumor; no solid areas or papillary excrescences; the adjacent tissue displayed yellowish areas (arrows).
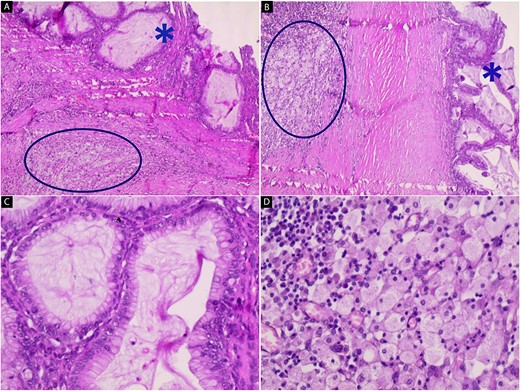
Hematoxylin and eosin stain (A–D); microscopic images of mass; (A and B) the low-power magnification reveals cystic glands lined by a single layer of non-ciliated, mucin-secreting, columnar epithelium without stromal invasion (star) with subepithelial fibrosis, foamy macrophages, and chronic inflammatory infiltrates (circle) (40x and 100x); (C and D) the high-power magnification shows mucin-secreting columnar epithelium and foamy macrophages with chronic inflammatory infiltrates respectively (200x and 200x).
Discussion
Mucinous cystadenomas are a common benign neoplasm of the ovaries that can grow much larger than other adnexal masses; they are recognized as precursors of ovarian cancer and may slowly transform into borderline tumors and invasive ovarian cancer [5], but the prognosis is usually good with a high survival rate when treated timely [6]. It is reported to occur in middle-aged women and may occur in younger age [3]. The resultant signs and symptoms are often secondary to the large size of the mass and may include pain, abdominal or pelvic fullness, or a palpable mass [7], as observed in our patient. Other complications of mucinous cystadenoma such as torsion, rupture, and hemorrhage may occur [7]. The clinical signs and symptoms are nonspecific, but the large size alone at physical examination is suggestive of a mucinous histologic type [7]. On gross appearance, mucinous tumors are characterized by cysts of variable sizes without surface invasion. Only 10% of primary mucinous cystadenoma is bilateral [3]. In our case, the tumor was unilateral, affecting the left ovary, and the cyst was filled with thick gelatinous mucoid. Treatment of mucinous cystadenoma is laparotomy or laparoscopic surgery depending on various factors like size of the cyst, age of the patient, parity, clinical presentation, etc. [6]. In our case, the patient underwent exploratory laparotomy. Xanthogranulomatous oophoritis on the other hand is a chronic uncommon inflammation characterized by the destruction of the tissues of the organ involved and replacement by chronic inflammatory cells such as lymphocytes, plasma cells, occasional neutrophils with or without multinucleated or touton giant cells [1]. Most commonly, females of reproductive age group 23–72 years are affected [2]. The exact etiology is not known but the theories of abnormality in lipid metabolism, infection, ineffective antibiotic therapy, and ineffective clearance of bacteria by phagocytes are proposed [8]. Infection with organisms like Proteus, Escherichia coli Bacteroides fragilis, Actinomyces, and Staphylococcus aureus are reported as probable causative organisms in studies. Uterine artery embolization and glove dusting powder are also hypothesized to cause the pathology [8]. Patients usually present with a long-standing history of pelvic inflammatory disease and suffer symptoms like anorexia, fever, and lower abdominal pain. There is a long history of antibiotic intake as well [1]. Our patient had an intermittent fever with lower abdominal pain and a history of taking antibiotics without getting better. Xanthogranulomatous oophoritis is rare but has been reported in the literature, only 15 cases involving the female genital tract are reported to date with few cases involving the ovary and reported from India [2]. Kunakemakorn was the first to report inflammatory pseudotumor in the pelvis in serosa of the uterus, left fallopian tube, and ovary [9], but its occurrence in conjunction with a mucinous cystadenoma is unprecedented. The pathogenesis of xanthogranulomatous inflammation remains unclear, and its association with mucinous cystadenoma raises intriguing questions. It is possible that chronic inflammation within the ovarian tissue, secondary to the mucinous cystadenoma, triggered the development of xanthogranulomatous changes. Alternatively, these two entities may share common etiological factors that predispose the ovary to both neoplastic and inflammatory processes. The coexistence of mucinous cystadenoma and xanthogranulomatous oophoritis in our patient represented a distinct pathological association. To our knowledge, this is the first reported case of such a combination, highlighting the rarity and complexity of ovarian pathology.
Conclusion
This case report documents a unique presentation of mucinous cystadenoma of the ovary with concurrent xanthogranulomatous oophoritis, a combination never before reported in the medical literature. The rarity of both entities and their simultaneous occurrence in the same patient makes this case noteworthy. The diagnostic and management challenges encountered underscore the importance of a multidisciplinary approach involving gynecologists, pathologists, and radiologists in the evaluation of complex ovarian pathology. The underlying etiological factors linking mucinous cystadenoma and xanthogranulomatous oophoritis remain elusive, warranting further research to elucidate the pathophysiological mechanisms involved. As the medical community encounters rare and unprecedented cases, comprehensive documentation and dissemination of such findings are crucial for expanding our understanding of ovarian pathology and guiding future clinical management strategies. This case serves as a valuable addition to the existing body of knowledge and prompts clinicians to consider unusual coexisting pathologies in the evaluation of ovarian masses.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding
None declared.
Consent
Informed and written consent from the patient was taken prior to publication.
Guarantor
Moatasem Hussein Al-janabi.



