-
PDF
- Split View
-
Views
-
Cite
Cite
Tahira Riaz, Saood Ahmed Riaz, A case of Lemmel’s syndrome: a rare cause of non-neoplastic obstruction of the biliary tract, Journal of Surgical Case Reports, Volume 2024, Issue 12, December 2024, rjae693, https://doi.org/10.1093/jscr/rjae693
Close - Share Icon Share
Abstract
We report the case of a 77-year-old woman with jaundice but no evidence of choledocholithiasis or other alterations of the biliary tree except for a duodenal diverticulum. Lemmel’s syndrome was diagnosed and an endoscopic sphincterotomy with stenting was performed. Lemmel’s syndrome is a rare disease that must be considered as a cause of obstructive jaundice.
Introduction
Duodenum is second most frequent location for diverticulum after colon and literature review shows that 5% and 10% of the general population may develop them [1]. Patients are initially asymptomatic but when they become symptomatic the symptoms present in the form of abdominal pain, nausea and jaundice. Obstructive jaundice caused by Peri-ampullary duodenal diverticulum (PAD) in the absence of choledocholithiasis or neoplasm.
Lemmel’s syndrome-described by Dr. Gerhard Lemmel in 1934 is also known as intermittent obstructive jaundice. It is defined as a pseudodiverticula without a muscle layer. Literature review shows only few published cases, and this pathology has yet to be thoroughly studied. PAD is acquired extraluminal outpouching of the duodenal wall located within 2–3 cm to ampulla of Vater [1].
In general, most patients have jaundice due to direct bilirubin, acute abdominal pain, and cholangitis. Patients may also present with postprandial epigastric pain and a sensation of fullness.
The exact pathophysiology is unknown but it is postulated that it is related to increased pressure, mechanical obstruction, distortion of the distal portion of the common bile duct and the pancreatic duct or to dysfunction or spasms of the sphincter of Oddi. All this favors reflux of the duodenal contents and intestinal bacteria into the common bile duct and the pancreatic duct and to biliary stasis which ultimately produces the symptoms caused by obstructive pathology of the intraduodenal distal third of the bile duct [2].
Case description
We present a case of a 70 year old female who presented in emergency department with a 3 day history of constant abdominal pain in epigastrium and subsequent migrating to right upper quadrant (RUQ) with nausea, anorexia, and jaundice. Her background history was significant for laparoscopic cholecystectomy (1994), right mastectomy and SLNB: IDC T2N0M0 grade 2 (2013), lararoscopic adhesiolysis (SBO; 2014) and hypertension. Her general physical examination revealed an obese lady with BMI of 33, mild scleral icterus, RUQ tenderness with negative Murphy’s sign. Her inital labs were Hb 12.7, WBC 14.3, CRP 160, amylase 51, lactate 1.1, liver function tests Tot. Bil 58.5, Direct 52.9, alkaline phosphatase (ALP) 167, gamma-glutamyl transferase (GGT) 221, and alanine transaminase (ALT) 99. These blood tests were followed by an ultrasound GB which showed no biliary dilatation. We booked an magnetic resonance cholangiopancreatography (MRCP) which also showed no evidence of choledocholithiasis. Dilatation of common bile duct (CBD) measuring 12 mm, pancreas is poorly visualized, low signals at pancreatic head- suggesting a CT AP for clarification. CT AP showed a low signal adjacent to pancreatic head representing a large duodenal diverticulum with resolved intra-and extra-hepatic biliary dilatation highlighted on MRCP and a normal CBD (Fig. 1).
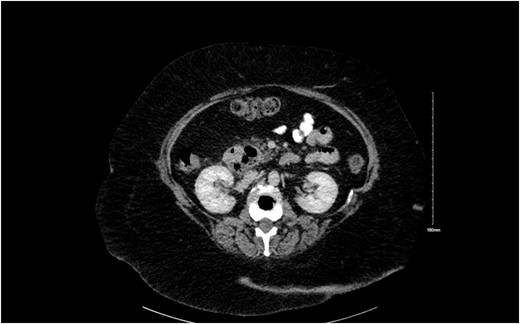
She was resuscitated prior to endoscopic retrograde cholangiopancreaticography (ERCP). Post-ERCP and stenting she was well clinically and biochemically. She was discharged home in a physically fit condition.
Discussion and conclusion
Duodenal diverticulum though rare usually occurs in 2–3 cm to ampulla of Vater. It is usually asymptomatic, but in 1%–2% patients can cause acute abdominal pain due to an extrinsic obstruction of common bile duct or pancreatic duct [3] as shown in Fig. 2.
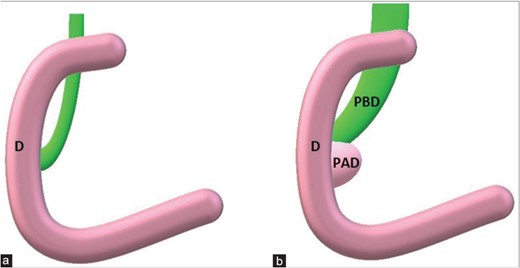
(a) Principal bile duct normally between 4 and 8 mm in maximum diameter. (b) When a periampullary duodenal diverticulum is present, its extrinsic compression could enlarge periampullary duodenal diverticulum till obstructive jaundice.
A clinically high suspicion of duodenal diverticula should be kept in mind for unknown case of jaundice/abdominal pain or other vague symptoms when no clear cause is identified. With the advent of CT, which is reliable, non-invasive, it has become the diagnostic investigation of choice [4]. The treatment options when conservative management fails include:
Surgical resection (diverticulectomy)
Endoscopic sphincterotomy
Papillary balloon dilatation
Conflict of interest statement
None declared.
Funding
None declared.



