-
PDF
- Split View
-
Views
-
Cite
Cite
Martin Rutegård, Anders Gerdin, Jannice Forssell, Olle Sjöström, Andreas Söderström, Petrus Vinnars, Robotic low anterior resection with complete splenic flexure mobilization and defunctioning left-sided loop colostomy: a case series, Journal of Surgical Case Reports, Volume 2024, Issue 1, January 2024, rjad709, https://doi.org/10.1093/jscr/rjad709
Close - Share Icon Share
Abstract
A defunctioning stoma is used to alleviate the consequences of anastomotic leakage after low anterior resection for rectal cancer. A loop ileostomy is often preferred but may lead to dehydration and kidney injury. Here, we present a case series for an alternative: the left-sided loop colostomy. A convenience sample of four patients underwent robotic low anterior resection for rectal cancer. A complete splenic flexure mobilization and a total mesorectal excision were performed. To defunction the anastomosis, the redundant left colon was brought up to a stoma site in the left iliac fossa and matured as a loop colostomy. Two patients experienced minor stoma leaks and one also had a small prolapse, while all patients had their colostomies reversed on average 7 months after surgery without complications. There were no dehydration episodes and creatinine levels remained within baseline levels at end of follow-up (on average 18 months).
Introduction
Anterior resection with an anastomosis is the main curative option for upper and mid rectal cancer, following the principles of total mesorectal excision [1]. Anastomotic leakage is a common event, ranging from 10 to 20% in population-based data [2, 3]. Several randomized controlled trials have shown that a defunctioning stoma is an effective method to reduce the incidence of early symptomatic leakage [4]. The typical defunctioning stoma is a loop ileostomy [5], due to the ease of fashioning and reversing such a stoma. However, a loop ileostomy may cause high-stoma output, dehydration, and even kidney injury [6]. The main proven alternative is a loop transverse colostomy, though this stoma type typically requires additional dissection and is more prone to prolapse [7]. Quite a few temporarily intended stomas are never reversed, chiefly due to disseminated disease or postoperative complications [8]. This would be an argument in favour of loop colostomies, as colostomies are physiologically more suited for permanence than ileostomies, hence negating the need for additional surgery.
Traditionally, a splenic flexure mobilization was considered an integral part of a low anterior resection to ensure a tension-free anastomosis [9], but this dogma has been questioned and it has been difficult to prove an influence on leak rates [10]. This procedure is often considered more difficult in minimally invasive surgery, especially with earlier generations of surgical robotics. Later generations have made this manoeuvre easier, and some robotic surgeons propose an approach where the splenic flexure mobilization is considered the first step in a low anterior resection, effectively making mobilization routine [11]. After such a complete splenic flexure mobilization, we have typically found that there is a considerable amount of redundant left colon even after resection of the specimen, even if this includes the entirety of the sigmoid colon. For appropriate cases, we have used this redundance to fashion a left-sided loop colostomy in the left iliac fossa, after construction of the anastomosis. Our surgical procedure and experience with four cases are described below.
Case series
Preoperative considerations
All patients had mechanical bowel preparation the day before surgery and were preoperatively marked for possible stoma sites in both the right and left iliac fossae by experienced stoma nurses. Prophylactic oral antibiotics—metronidazole and trimethoprime-sulfametoxazole—were given in the morning of surgery, with an additional dose of intravenous trimethoprime-sulfametoxazole after 4 hours of surgery. A spinal anaesthetic was provided before induction of general anaesthesia.
Low anterior resection with complete splenic flexure mobilization
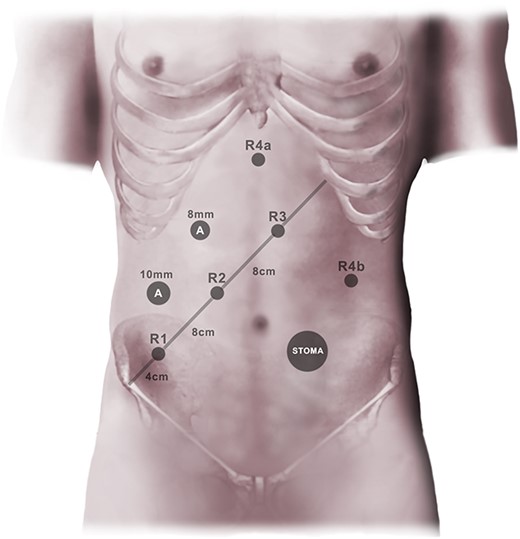
A standardized approach for all low anterior resections was employed, using the da Vinci® Xi robot. A mini-laparotomy was made in the left flank to establish pneumoperitoneum, using a 12 mm balloon port. A line from the right anterior superior iliac spine to the mid left costal margin was drawn. Three 8 mm robotic ports were inserted at this line, beginning 3–4 cm from the iliac spine, with intervals of 8 cm. Two additional 8 mm robotic ports were placed: one just to the right of the midline in the epigastrium, one in the left flank (Fig. 1); an 8 mm Airseal® port constituted the last port, enabling two-handed surgical assistance. The patient was placed in a Trendelenburg position with 22°head down tilt and 12° rotation (left side up). Laparoscopic instruments were used to position bowel and greater omentum, making sure that the duodenal junction was at least partly visible, employing gauze if need be. The robotic system was subsequently docked, using the first three ports and the epigastric port. A 30° camera was used in port two, along with a Prograsp forceps in the epigastric port, bipolar grasper in port three, and monopolar curved scissors (da Vinci® Surgical System, Intuitive Surgical, Sunnyvale, CA) in port three. The first step consisted of identifying the inferior mesenteric vein and dissecting between the mesocolon and Gerota’s fascia. Dissection ensued in this embryological plane until bowel was encountered laterally and further cranial and caudal dissection became cumbersome. At this point, the vein was divided between clips as close to the inferior border of the pancreas as possible, sharply releasing the duodenal junction first if required. The anterior aspect of the pancreas was subsequently identified and released from the transverse mesentery, allowing entry into the lesser sac. This opening was then expanded along the anterior and lateral border of the pancreas, while avoiding the hilum of the spleen. Gauze was placed in this space for future reference. The transverse colon was then brought caudally, and the greater omentum released from the mid transverse colon to the splenic flexure. Thereafter, attention was given to the inferior mesenteric artery, beginning with a medial incision that was developed cranially to the vascular pedicle along the embryological plane. The artery was divided between clips close to the aorta before the left colic artery take-off. With traction of the bowel towards the midline, the lateral attachments were subsequently released from the pelvic inlet to the splenic flexure, ensuring complete mobilization distal to the middle colic arteries. At this point, the epigastric robotic arm was repositioned to the port in the left flank, as a total mesorectal excision was then performed down to the pelvic floor. After rectal washout, the rectal tube was divided with two or three firings of a 45 mm robotic stapler. The descending colon mesentery was then divided from the vascular pedicle origin to the bowel wall, and the aboral end of the specimen was grasped with a laparoscopic grasper. After docking off the robot, a 4–6 cm Pfannenstiel incision was made, and a wound retractor was inserted. The bowel was exteriorized, and the specimen transected, with pulsatile bleeding ensuring good marginal artery perfusion. A side-to-end configuration was prepared using the anvil of a 29 mm circular powered stapler, where the anvil was brought out at the antimesenteric side. A linear stapler was used to close the end of the bowel and the staple line was oversewn with a resorbable monofilament running suture. The bowel was returned to the abdomen and conventional laparoscopy ensued. A double-stapled side-to-end anastomosis was subsequently performed, with complete stapler doughnuts and negative air leak tests as a result (Fig. 2).
Maturation of loop colostomy and closure
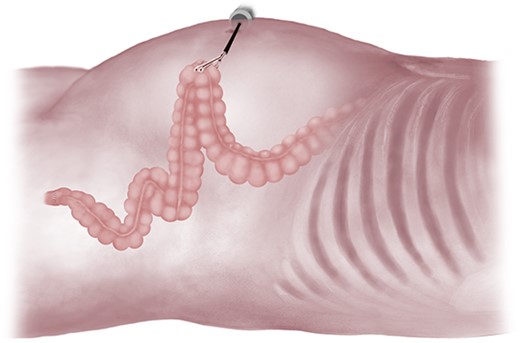
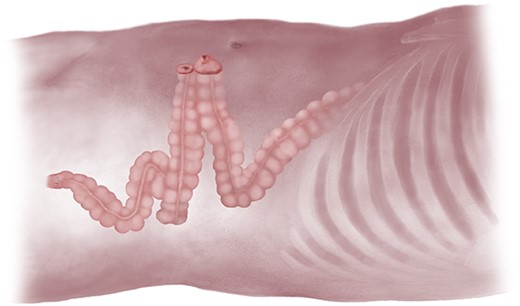
At this stage, a long redundant left colon was noted, despite specimen resection and construction of an anastomosis. This bowel loop was tested for length and found in all cases to be easily brought up to the abdominal wall without any tension on the anastomosis itself (Fig. 3). Insufflation was then stopped and a 4–5 cm circular incision in the left iliac fossa was made through skin and down to fascia. A similarly sized cruciate incision was then performed in the rectus sheath, the rectus muscle fibres were bluntly separated, the abdomen was entered, and the left colon exteriorized without any tension. The other fascial and skin incisions were closed with resorbable sutures, after which dressings were applied. A loop colostomy was subsequently matured, everting the oral limb ~1 cm above the skin edges with three-point resorbable monofilament sutures, while sewing the aboral limb flush to skin with two-point sutures. Stoma appliances were attached, completing the operation (Fig. 4).
Postoperative results
In a convenience sample, two men and two women were operated as described above during 2022 at Umeå University Hospital. Mean age was 50.3 years and the average body mass index was 26.7 kg/m2. All patients had an American Society of Anesthesiologists’ fitness grade of II. Initial staging revealed two localized tumours, while two were locally advanced, necessitating short-course radiotherapy followed by chemotherapy according to the RAPIDO trial [12]. The mean intraoperative bleeding was 63 ml, while the average operative time was 439 minutes. None of the patients experienced postoperative complications and mean length of stay after the operative procedure was 5.5 days. All tumours were radically excised and after discussion at the local colorectal multidisciplinary team meeting, two of the patients were referred for adjuvant chemotherapy.
At the end of follow-up (on average 18 months), no readmissions for high-stoma output or dehydration were noted; serum creatinine levels were similar to baseline for all patients. There were no cases of stoma prolapse or retraction. Two patients had minor incidents of stoma leak and dermatitis, while one of these also had a small stoma prolapse; this was all resolved with the help of the stoma nurse who supplied appropriate dressings.
Stoma reversals were performed on average 7 months after the index procedure. They were all performed through a local procedure without the need for laparotomy. The loop colostomy was mobilized down to fascia and released using sharp dissection. The stoma edges were trimmed to fresh tissue but no formal bowel resection was required. The enterotomy was then closed with a resorbable monofilament running suture. The sheath was closed with a running suture, while the skin was sutured with a purse-string. No complications were noted for any of the patients and they were discharged after an average of 3.5 days. Low anterior resection syndrome (LARS) [13] was assessed in three patients on average 4.2 months after stoma reversal, with a mean LARS score of 29.
Ultimately, two patients had developed metastases; one had had a thoracoscopic lung metastasectomy, while one patient had developed disseminated pulmonary metastases and received palliative chemotherapy.
Discussion
We have described four cases of robotic low anterior resection for rectal cancer with a complete splenic flexure mobilization, using the resulting redundant left colon as a loop colostomy. No postoperative complications were noted from the index procedure nor the stoma reversal, while minor stoma-related problems such as stoma leakage and a small prolapse were registered. These data are promising, as a left colostomy would in theory have several potential advantages compared with the prevailing loop ileostomy: in case of no stoma reversal due to e.g. tumour dissemination or other concurrent disease, a colostomy placed where as much physiological bowel length as possible is preserved seems intuitively preferable. This is also to the patient’s benefit while waiting for stoma reversal, possibly negating the dehydration episodes commonly seen with loop ileostomies.
The main concern with this described stoma type is the risk of colonic ischaemia, as the stoma and the anastomosis are dependent on the marginal artery. Theoretically, the artery could be damaged during direct manipulation of the bowel or mesentery, but compression from the bowel wall within the stoma could also be an issue. In addition, the stoma reversal procedure itself might induce tissue damage, potentially risking the aboral limb to the anastomosis. We did not notice any such events in the present series, and any such event would likely make us abandon this technique. We do believe that utmost caution must be taken in not grasping the mesenteric border and that case selection is important, as a sizable layer of subcutaneous fat in the abdominal wall might jeopardize the marginal artery by compression; such a selection might have been reflected by the relatively low body mass index seen in our case series. Likewise, the stoma aperture must not under any circumstance be narrow. Nevertheless, we did not experience any parastomal hernias before or incisional hernias after stoma reversals. In accordance with the published literature on temporary loop stomas, these loop colostomies did not seem to be related to dehydration and we did not observe any major postoperative complications after stoma reversal, where a previous meta-analysis has concluded that only surgical site infections are increased after loop colostomy reversal [7]. Interestingly, there was just one case of a small prolapse; left-sided colostomies might be less prone to prolapse, as there should not be much colonic length left in any direction inside the abdomen.
Conclusions
The initial experience of four patients provided with a defunctioning left-sided loop colostomy is promising. The potential advantages are numerous compared with a loop ileostomy, not least to avoid kidney injury, and to have a physiologically superior stoma for the patient in the long run for those where a stoma reversal is never realized. However, prospective cohorts are needed to properly investigate the safety and efficacy of this approach, where the theoretical risk of compromising the marginal artery is a concern.
Acknowledgements
Dr Erling Petersen provided the medical illustrations in the manuscript.
Conflict of interest statement
None declared.
Funding
A grant from Region Västerbotten (HSN 530-2022) for stoma research supported the study. The funding body has had no influence on any part of the study.
Data availability
Data can be shared upon reasonable request.
Author contributions
M.R. conceived the study, performed the operative procedures, and wrote the manuscript draft. A.G., P.V., and J.F. participated in the operative procedures and reviewed the manuscript. O.S. and A.S. conceived the study and reviewed the manuscript. All authors approved the final version.
Ethics approval and consent to participate
The study was approved by the Swedish Ethical Review Authority (Dnr 2023-05683-01).
References
- loop ileostomy
- dehydration
- creatinine
- anastomosis, surgical
- follow-up
- ilium
- stomas
- surgical procedures, operative
- colostomy procedure
- surgery specialty
- rectal carcinoma
- renal trauma
- low anterior resection of rectum
- mesorectal excision, total
- rectosigmoidectomy
- splenic flexure
- descending colon
- loop colostomy



