-
PDF
- Split View
-
Views
-
Cite
Cite
Olivia Julian, Kailyn Wilcox, Davek Sharma, Kathleen Lamb, Robert Luo, Hong Zheng, Renganaden Sooppan, Amir Behnam, Viability of the rectus femoris muscle flap for groin wound coverage after ligation of proximal inflow, Journal of Surgical Case Reports, Volume 2024, Issue 1, January 2024, rjad306, https://doi.org/10.1093/jscr/rjad306
Close - Share Icon Share
Abstract
Lower extremity revascularization via groin incisions can be complicated by wound dehiscence associated with infection, seroma and femoral vessel exposure. This may require additional surgical debridement and coverage of vascular structures and grafts. The pedicled rectus femoris muscle flap (RFF) has both bulk and a large arc of rotation, making it useful for reconstruction. Its main pedicle is the descending branch of the lateral femoral circumflex artery (DLFCA), a branch of the profunda femoris artery. One could anticipate that ligation of more proximal vasculature could lead to ischemia of the RFF. We present two patients who each underwent vascular surgery involving the common femoral artery and subsequent reconstruction utilizing a pedicled RFF. Both patients then required additional vascular procedures involving the ligation of inflow vessels proximal to the DLFCA. The flaps remained viable, demonstrating the rich collateralization of blood supply that occurs in vascular disease patients.
Introduction
Postoperative wound complications such as infections, seromas and wound dehiscence may occur following lower extremity revascularization procedures that involve a groin incision. Various sources in the literature cite infection rates anywhere from 4 to 40%, with high associated amputation and mortality rates [1, 2]. Poor blood supply and associated medical comorbidities in peripheral vascular disease patients can impair wound healing, leading to postoperative complications and a return to the operating room for further management, including muscle flap reconstruction.
Muscle flaps are effective for covering exposed vascular structures including native vessels and grafts, obliterating dead space, increasing regional blood supply and decreasing bacterial colonization at the wound [3]. Commonly utilized muscle flaps include the sartorius, gracilis, rectus abdominis and rectus femoris. A pedicled rectus femoris muscle flap (RFF) is suitable because of its size, bulk and ability to be mobilized on its long pedicle; its sacrifice usually results in minimal morbidity, most notably the loss of the terminal 15–20 degrees of knee extension. As a Mathes and Nahai Type II muscle flap, it has one dominant pedicle and one or more minor pedicles [4]. The RFF’s main pedicle is the descending branch of the lateral femoral circumflex artery (DLFCA), a branch of the profunda femoris artery (PFA), also known as the deep artery of the thigh. The common femoral artery (CFA) bifurcates in the proximal thigh into the PFA and superficial femoral artery (SFA) distally (Fig. 1) [5].
![Femoral artery and its branches [5].](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2024/1/10.1093_jscr_rjad306/3/m_rjad306f1.jpeg?Expires=1773872230&Signature=DPg07-rMCxvAifg54Fq4SXOkRiFZ-XZ4DDOJNhviMxilF6lcLhbtvxy3Jyg9~9GdmQdhEr4YsPK3ovHr7YyjjIfddcEhL4Wy5Cg5VLumUG-nr9BywwZbo2arkaeOf16ma3LfY-MK7RXwpWQ2lO9jPB26QfeUtdpGSJhNn3fjQGrG5J9FGQS4uXm542hzIl8nUCyn~2qLh9qaEiG4omBSzq6hNREstrC1bSX5tu3ZlCeV-u5LQikSUB3CL1g1wUenfywZk3S52hr14dMFvsBq2UeJcikdHJ0iq34dKYh0ZAGdAfPc~Ns7P1xc-fTisRRHMw2PLIHn9XFmManDM5UH8g__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
One could anticipate that a disruption in the vasculature proximal to the pedicle could lead to poor perfusion and compromised viability of the muscle flap. We present two patients who underwent extensive lower extremity vascular surgery over the groin. In both cases, plastic and reconstructive surgery (PRS) was consulted because of postoperative wound complications requiring revisional and reconstructive surgery. In both instances, the rectus femoris muscle was harvested, transposed and inset with a split-thickness skin graft (STSG) and negative pressure wound therapy vacuum (VAC) over the groin wound and associated vascular structures.
Days after successful muscle flap reconstruction, both patients returned to the operating room for further vascular procedures. In both cases, the CFA was ligated. Even after proximal vascular ligation, the RFFs remained viable, suggesting a robust collateral blood supply to the dissected rectus femoris muscle via the DLFCA. To our knowledge, this report is the first description of RFF viability under such circumstances. Our results highlight its utility in complex groin reconstruction when extensive vascular disease is present.
Case descriptions
Case 1
This patient was a 70-year-old male with past medical history including peripheral vascular disease, coronary artery disease, hypertension, chronic obstructive pulmonary disease, hyperlipidemia and a 60-pack-year smoking history. He presented to the emergency department because of right leg pain and was diagnosed with an acute aortoiliac occlusion with right lower extremity Rutherford IIb ischemia. Vascular surgery promptly completed a right axillary to right CFA prosthetic bypass graft and endarterectomy given his extensive medical comorbidities. Postoperatively, the patient developed a right femoral hematoma because of the need for anticoagulation for acute thrombosis, which was associated with wound breakdown and infection.
After operative hematoma evacuation and debridement, microbiology culture sampling and wash out, the PRS team utilized a right pedicled RFF with STSG for coverage of the exposed graft (Fig. 2A). Unfortunately, the vascular graft ultimately had to be explanted in a third operation because of culture results indicating infection; at that time, vascular surgery completed revisional aorto-above knee popliteal artery obturator bypass with a cryo-artery, ligation of the right CFA and patch angioplasty of the distal right CFA, just proximal to the bifurcation of the PFA and SFA. There was a biphasic doppler signal in the proximal SFA and PFA despite ligation of arterial inflow because of retrograde flow via the bypass graft. The RFF was lifted proximally and reapplied but remained viable throughout.
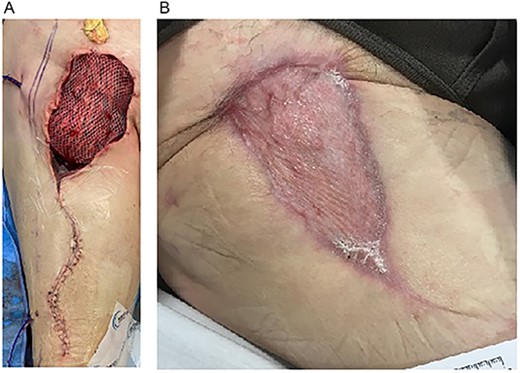
(A) Inset of right RFF-STSG over right groin defect. (B) Four months postoperative. Well-healed RFF-STSG over right groin.
After a 6-week hospitalization including rehabilitation, the patient was discharged home and ultimately completed a 6-week course of intravenous antibiotics (Fig. 2B). Eleven weeks postoperatively and in outpatient follow up, the patient was doing well; the groin wound-RFF reconstruction was healing without compromise.
Case 2
This patient was an 82-year-old male with past medical history including peripheral vascular disease, hypertension, coronary artery disease, transient ischemic attack, diabetes mellitus, chronic right foot osteomyelitis and a 46-pack-year smoking history. He had an extensive vascular surgical history, including right carotid endarterectomy and left CFA endarterectomy with femoral-popliteal bypass.
The patient underwent an elective right CFA endarterectomy with retrograde external iliac stent placement. Postoperatively, he developed a necrotizing wound infection likely related to seeding of digital osteomyelitis, complicated by bacterial endocarditis. Vascular surgery explanted the bovine pericardial patch angioplasty and replaced it with native great saphenous vein. After multiple groin washouts and VAC applications, PRS completed an RFF reconstruction of the resultant groin defect with coverage of the femoral vessels (Fig. 3A–C).
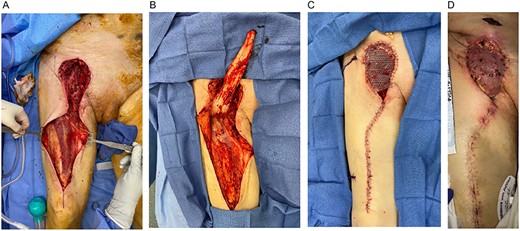
(A) Right groin defect and rectus femoris muscle. (B) Right groin defect with RFF elevated proximally to demonstrate adequate groin coverage. (C) Right groin reconstruction with RFF with STSG. (D) Two weeks postoperative; RFF-STSG.
Two weeks postoperatively and after discharge to a rehabilitation facility, the patient presented to the emergency department because of bleeding from the right groin (Fig. 3D). The patient was diagnosed with an enlarging right CFA pseudoaneurysm and was subsequently taken back to the operating room for balloon occlusion of the right external iliac artery and ligation of the CFA, PFA and SFA. Arteriogram was performed, demonstrating filling of the PFA via collaterals. Concurrently, PRS and vascular surgery elevated the previously transposed RFF-STSG to allow for appropriate vascular exposure and then re-inset it for groin coverage-reconstruction. The RFF remained viable despite ligation of the proximal blood supply. Ultimately, the patient passed away six weeks postoperatively because of respiratory failure.
Discussion
Patients undergoing vascular surgery have significant rates of wound healing complications, infections and limb amputations following infra-inguinal revascularization. When considering this patient population and its associated medical morbidities, these rates are not surprising. It is imperative to avoid wound breakdown and exposure or infection of vascular structures—native or prosthetic. Muscle flaps are effective for groin wound reconstruction after vascular surgery associated with such complications; they provide bulky viable tissue to cover critical vascular structures and provide additional regional blood supply to promote healing and the delivery of antibiotics.
Historically, the sartorius muscle flap (SMF) was commonly utilized for groin wound reconstruction in vascular surgery because of its proximity to the wound, ease of dissection and limited donor site morbidity. However, the SMF has limited volume-bulk and may be unable to obliterate dead space defects associated with postoperative seromas or hematomas; its blood supply, the SFA, is often involved in atherosclerotic disease [2], and is a Mathes Type IV muscle flap with a segmental blood supply, meaning that flap viability can be compromised with sequential perforator ligation in an attempt to significantly mobilize the flap. As an acceptable alternative, the RFF was first utilized by plastic surgeons in 1989 [1]. Its benefits include more muscle bulk to reconstruct large defects, a larger rotational arc, the relative sparing of its pedicle in vascular disease patients and minimal functional deficit after harvest.
A recent retrospective study compared outcomes using SMF versus RFF to cover groin wounds after vascular surgery and found comparable flap survival rates of 94% in the RFF group versus 90% in the SMF group [1]. Another review analyzed outcomes in vascular patients treated with RFF coverage and found a 96% 30-day survival rate, 93% salvage of the vascular graft and avoidance of major amputation in 20 of 24 affected limbs [2]. These results are comparable to previous findings, including those by Alkon et al. [3], who found that of 37 RFFs, there were no flap losses or donor site infections, and 94.6% of wounds healed after flap coverage. This group has even used the RFF prophylactically in high-risk vascular surgery patients who are likely to have postoperative wound complications with low rates of vascular graft loss [6]. Interestingly, the RFF has also been found to be the more cost-effective option as compared with the SMF, based on the differences in complication rates and cost-utility analysis [7].
Potential disadvantages of the RFF include an additional donor site incision, whereas the SMF can be harvested within the original vascular surgical site. Additionally, up to 28% loss of power and terminal extension of the knee is possible; however, findings suggest that this is reversible with postoperative physical therapy [3]. These potential complications highlight the importance of flap choice in various patient populations—an RFF may not be an appropriate choice in a young athlete but may be preferred in an older individual with vascular disease. The rectus femoris was chosen in both patients described above mainly because of surgeon preference, considering the large defect size, the ability of the RFF to rotate sufficiently and the extensive atherosclerotic disease present. After rehabilitation, our patients did not demonstrate any functional deficit in knee extension and they ambulated normally.
The cases presented are remarkable because the RFFs remained viable despite the need for further vascular interventions mandating re-elevation of the flap and more importantly ligation of vessels proximal to its blood supply. Their success raises the question of how the muscle flaps were able to maintain sufficient perfusion. It is well established that in the vascular disease population, collateralization occurs to improve blood flow in areas inadequately perfused by the diseased vessels [8]. This phenomenon may have contributed to RFF survival in our cases. These findings suggest that in vascular disease patients, the rectus femoris muscle has a robust blood supply fueled by not only its main pedicle but also a network of collateral blood supply that contributes to its viability. There may also have been a significant contribution from retrograde flow after ligation of the CFA. Post-ligation vascular studies may have confirmed this proposition but were not performed because of potential procedure morbidity and costs.
Conclusion
The RFF is an effective option for complex groin wound reconstruction in vascular surgery patients with postoperative surgical wound complications. Although donor site morbidity may include some degree of loss of knee extension and strength, it is an effective option for patients with extensive vascular disease and a large defect with or without infection. The RFF has a robust blood supply through its main pedicle, the DLFCA. The delineated cases are unique because both patients had further vascular interventions requiring flap re-elevation and CFA ligation. These cases act as preliminary evidence for the presumed role of collateralization on the viability of muscle flaps in vascular disease patients.
Future investigations could help surgeons better understand the vascular physiology of pedicled muscle flaps and how vascular disease and disruption of proximal blood supply affect flap viability. With such knowledge, both vascular and plastic and reconstructive surgeons could potentially direct better care of their shared patient population.
Conflict of interest statement
None declared.
Funding
None declared.
Data availability
No datasets were generated or analysed during the current study.



