-
PDF
- Split View
-
Views
-
Cite
Cite
Masatsugu Hiraki, Toshiya Tanaka, Hirofumi Sato, Shuusuke Miyake, Hiroshi Kubo, Yukio Shinkai, Eiji Sadashima, Kenji Kitahara, The analysis of fecal calprotectin as a diagnostic marker for anastomotic leakage after rectal cancer surgery: a pilot study, Journal of Surgical Case Reports, Volume 2023, Issue 7, July 2023, rjad432, https://doi.org/10.1093/jscr/rjad432
Close - Share Icon Share
Abstract
A prospective pilot study was conducted on 11 patients with rectal cancer to investigate fecal calprotectin (FC) as a diagnostic tool for detecting anastomotic leakage (AL) after low anterior resection. Among the 11 patients, 1 patient (9.1%) experienced AL (Clavien-Dindo Grade IIIa). During the post-operative course until post-operative day (POD) 5, the white blood cell count of the patient with AL was within the normal range. The C-reactive protein level in the AL and non-AL groups showed a similar time course. On the other hand, the FC level in patient with AL dramatically increased on POD5, while the FC level of the non-AL group remained relatively stable. There was no significant correlation between the preoperative FC level and the tumor circumference rate, tumor size, depth of invasion or stage. This pilot study showed the possibility of FC as a useful diagnostic tool for the detection of AL after low anterior resection for rectal cancer.
INTRODUCTION
Anastomotic leakage (AL) is a severe complication that leads to bacterial infection, including sepsis, abdominal abscess and panperitonitis, resulting in an emergency operation and stoma creation. In addition, AL can lead to oncological disadvantages such as local recurrence and a poor prognosis [1, 2]. Despite the introduction of novel techniques and devices to prevent the AL, including transanal decompression tube [3, 4], polyglycolic acid sheet for the reinforcement of staple lines [5, 6], powered circular staplers [7] and triple-layered circular staplers [8], the incidence of AL in recent reports was still 4.6–15.8% [4, 7–9]. AL is diagnosed based on the findings of physical examinations, such as the presence of abdominal pain, body temperature elevation and the discharge of feces or pus from the abdominal drain, and blood test results. However, computed tomography (CT) or enema examination is often required to make a definitive diagnosis, and some cases are still difficult to diagnose. Therefore, more sensitive and accurate diagnostic markers or tools are needed to aid in the diagnosis of AL.
Calprotectin is a calcium-binding cytosolic protein complex that consists of S100A8 and S100A9 [10, 11]. It is found in neutrophilic granulocytes, monocytes and macrophages. The S100A8/S100A9 complex controls the intracellular pathways of innate immune cells and allows the orchestration of the inflammatory response. Typically, patients with chronic inflammatory diseases of the intestine show increased fecal calprotectin (FC) concentrations, partly because neutrophilic inflammation is an aspect of such diseases [12, 13]. Therefore, the examination of FC is widely accepted for the non-invasive assessment of inflammatory bowel disease (IBD) [13]. In this study, we investigated whether FC could be applied as a diagnostic tool for AL. FC was selected because AL results in local inflammation at the anastomotic site due to dehiscence and leakage of the anastomotic site and the leakage of feces outside the digestive tract; thus, AL might be diagnosed based on the FC level. The aim of this study is to investigate the possible application of FC as a diagnostic tool for the detection of AL after low anterior resection for rectal cancer.
CASE SERIES
Materials and methods
Patients
A prospective pilot study was conducted for patients who underwent laparoscopic low anterior resection for rectal cancer in a single institution at the Department of Surgery, Saga Medical Center Koseikan, between July 2020 and December 2021. All patients and their families were fully informed about the surgical procedures in order to obtain their written consent. Written informed consent and broad consent to participate in this study were obtained. The medical records of all patients were reviewed in detail. The medical ethics committee of Saga Medical Center Koseikan reviewed and approved this study design (permission number: 20-06-01-01). During the study period, 30 patients received low anterior resection in our institution, and written informed consent was obtained and sample collection could be performed in 19 patients. Among the 19 patients, 8 patients had missing data due to failure to collect feces samples. The remaining 11 patients were eligible for inclusion in this study.
Patient management and surgical procedure
Rectal cancer was confirmed preoperatively by colonoscopy and a pathological examination. In all patients, the presence of IBD was ruled out by endoscopy. Mechanical bowel preparation was performed using magnesium citrate with sodium picosulfate oral solution before the operation. All patients underwent laparoscopic low anterior resection, which is defined as tumor-specific mesorectal excision or total mesorectal excision, with adequate lymphadenectomy, following colorectal anastomosis using the double stapling technique. The decision to create a protective ileostomy was made based on the condition of the bowel and/or the surgeon’s technical evaluation of the quality of anastomosis. A 10-mm duple drain was used as a transanal decompression tube. It was gently inserted into the anus and placed on the oral side of the anastomotic site. On post-operative day (POD) 5, the transanal decompression tube was removed and the patient started receiving liquid food. Feces samples were obtained preoperatively and on POD1, POD3 and POD5. Post-operative fecal samples were collected from the transanal decompression tube or from diapers. AL was confirmed by CT and/or enema examination with water-soluble contrast fluid, following complications of abdominal pain, fever elevation or the discharge of feces or pus from the abdominal drain. The pathological tumor stage was classified according to the seventh edition of the UICC-TNM classification.
Statistical analyses
Continuous variables were expressed as the median and range, while categorical variables were expressed as numbers. The association between the FC level and other clinicopathological factors was assessed using Spearman’s rank correlation coefficients (r) or the Mann–Whitney U test. P values of <0.05 were considered to be statistically significant. All analyses were conducted using the SPSS software program, version 25 (IBM Japan, Tokyo, Japan) and R version 4.2.2.
RESULTS
The characteristics of the patients are summarized in Table 1. Among the 11 patients who received laparoscopic low anterior resection for rectal cancer, 1 patient (9.1%) experienced AL (Clavien-Dindo Grade IIIa). Two patients underwent the creation of a protective ileostomy at the initial surgery due to the poor condition of the intestine on the oral side of the anastomotic site. A patient who experienced AL was included among the patients who underwent the creation of a protective ileostomy. The white blood cell count of the patient with AL remained within the normal range until POD5 (Fig. 1a). In addition, the C-reactive protein (CRP) level of this patient peaked on POD3, and improvement was seen on POD5 (Fig. 1b). On the other hand, the FC level of this patient showed a dramatic increase on POD5 (Fig. 1c). This patient was found to have AL on POD8. In the non-AL group, gradual improvement of the white blood cell count and CRP level was observed until POD5 (Fig. 1d and e). In addition, the FC level of the non-AL group remained relatively stable in comparison to the patients with AL (Fig. 1f).
| Characteristics . | n = 11 . |
|---|---|
| Sex (male: female) | 8:3 |
| Age (years: median (min, max)) | 74 (49, 90) |
| Preoperative bowel obstruction (yes: no) | 1:10 |
| Tumor location (upper: lower) | 9:2 |
| TNM stage (0–II: III, IV) | 7:4 |
| T category (T1, T2: T3, T4) | 4:7 |
| N category (negative: positive) | 7:4 |
| M category (negative: positive) | 10:1 |
| Maximum tumor size (mm: median (min, max)) | 45 (24, 85) |
| Circumference rate of the tumor (%: median (min, max)) | 64 (33, 100) |
| Diverting stoma (yes: no) | 2:9 |
| Characteristics . | n = 11 . |
|---|---|
| Sex (male: female) | 8:3 |
| Age (years: median (min, max)) | 74 (49, 90) |
| Preoperative bowel obstruction (yes: no) | 1:10 |
| Tumor location (upper: lower) | 9:2 |
| TNM stage (0–II: III, IV) | 7:4 |
| T category (T1, T2: T3, T4) | 4:7 |
| N category (negative: positive) | 7:4 |
| M category (negative: positive) | 10:1 |
| Maximum tumor size (mm: median (min, max)) | 45 (24, 85) |
| Circumference rate of the tumor (%: median (min, max)) | 64 (33, 100) |
| Diverting stoma (yes: no) | 2:9 |
| Characteristics . | n = 11 . |
|---|---|
| Sex (male: female) | 8:3 |
| Age (years: median (min, max)) | 74 (49, 90) |
| Preoperative bowel obstruction (yes: no) | 1:10 |
| Tumor location (upper: lower) | 9:2 |
| TNM stage (0–II: III, IV) | 7:4 |
| T category (T1, T2: T3, T4) | 4:7 |
| N category (negative: positive) | 7:4 |
| M category (negative: positive) | 10:1 |
| Maximum tumor size (mm: median (min, max)) | 45 (24, 85) |
| Circumference rate of the tumor (%: median (min, max)) | 64 (33, 100) |
| Diverting stoma (yes: no) | 2:9 |
| Characteristics . | n = 11 . |
|---|---|
| Sex (male: female) | 8:3 |
| Age (years: median (min, max)) | 74 (49, 90) |
| Preoperative bowel obstruction (yes: no) | 1:10 |
| Tumor location (upper: lower) | 9:2 |
| TNM stage (0–II: III, IV) | 7:4 |
| T category (T1, T2: T3, T4) | 4:7 |
| N category (negative: positive) | 7:4 |
| M category (negative: positive) | 10:1 |
| Maximum tumor size (mm: median (min, max)) | 45 (24, 85) |
| Circumference rate of the tumor (%: median (min, max)) | 64 (33, 100) |
| Diverting stoma (yes: no) | 2:9 |
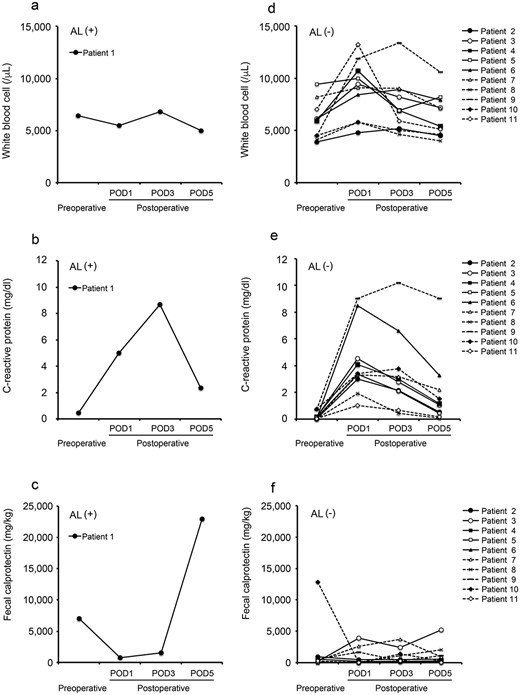
Perioperative values of inflammatory markers and FC in all patients; white blood cell count (a), CRP (b) and FC (c) in patient with AL; white blood cell count (d), CRP (e) and FC (f) in patient without AL.
There was no significant correlation between the preoperative FC level and the tumor circumference rate, tumor size, depth of invasion or stage (Fig. 2a–d).
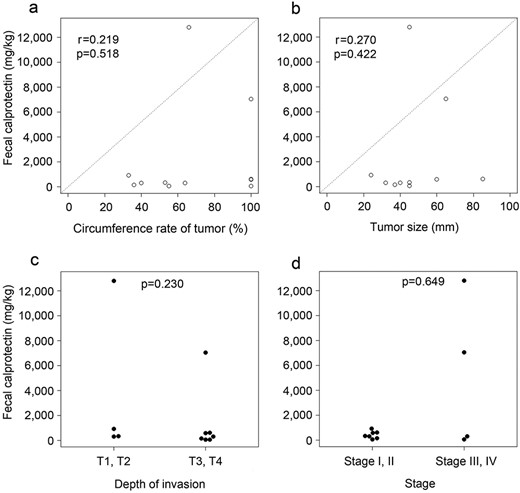
Correlation between clinicopathological factors; (a) circumference rate of tumor, (b) tumor size, (c) depth of invasion and (d) stage) and the preoperative FC level.
DISCUSSION
A recent systematic review of biomarkers for the detection of AL after colorectal surgery showed that CRP and procalcitonin (common inflammatory markers) could be reliable markers for the prediction of AL [14]. Other candidate markers, including oxidative stress markers and inflammatory biomarkers, such as interleukinj-6 or interleukin-10 from blood and/or drainage fluid samples have been also introduced [14]. However, these markers are still far from being applied in clinical practice. In actual clinical practice, the diagnosis of AL is sometimes difficult because patients with minor or occult AL sometimes show no abdominal pain or fever elevation, and their blood test parameters may also be within the normal range or show a normal course. Even if CT shows no evidence of AL, AL may be detected by an enema examination. Therefore, a more effective and convenient diagnostic tool is needed for the detection of the AL. In the normal post-operative course, inflammatory change due to surgery subsides with time after surgery. However, in the case of AL, the inflammation around the anastomotic site and intestinal tract does not subside and, conversely, calprotectin in the stool may increase because the inflammation becomes stronger. As a result, the signs of AL may be detected earlier.
We examined the FC level based on its potential application as a useful diagnostic tool for the detection of AL after rectal surgery. The reason why we applied this study for the low anterior resection is that constant fecal samples could be expected to be obtained through feces drained via a transanal decompression tube. To avoid AL, in recent practice, a transanal decompression tube is routinely placed as it can effectively reduce the incidence of AL [3, 4]. This approach is gaining widespread acceptance. In addition, the examination of FC is widely accepted in the clinical evaluation of IBD. In this study, we only experienced AL in one of the 11 patients. This patient underwent the creation of a protective ileostomy during surgery. Although the sample size is too small to conduct a proper analysis, interestingly, the patient with AL showed a dramatic increase in their FC level on POD5 despite the white blood cell count and CRP level peaking on POD3. Actually, the patient was found to have minor leakage (Clavien-Dindo IIIa) on POD8, which was confirmed by CT and an enema examination. This indicates that FC might be more sensitive for the diagnosis of AL in comparison to the white blood cell count or CRP level, which are used as standard inflammatory markers. We speculated that, due to protective ileostomy, the patient’s systemic inflammation may have been controlled and that, as a consequence, the white blood cell count and CRP level would not have increased as much. On the other hand, the FC level, which is a local inflammatory marker, was increased instead.
We also hypothesized that that the efficacy of FC as a local inflammatory marker could also be assessed by the evaluation of obstructive colitis. Colorectal cancer with a large tumor circumference leads to intestinal obstruction and/or obstructive colitis following fecal obstruction. Thickening of the colonic wall with edema and inflammation due to obstructive colitis could be a risk factor for AL after rectal surgery. However, our results showed no correlation between the circumference rate of tumor, tumor size or depth of invasion. Our study investigated a relatively small population. Therefore, it might be interesting to analyze the relationship between the FC level and the circumference rate of tumor and the intestinal condition in a larger population.
The present study was associated with several limitations. The study population was small and the study was conducted at a single institution. In addition, it is sometimes difficult to obtain fecal samples from a transanal decompression tube after preoperative mechanical preparation. Moreover, only patient with AL in our study population had received protective ileostomy, and stool from the anus had a very high concentration of mucus. Accordingly, it is possible that the calprotectin level measured in the stool would be more concentrated than that in a patient with normal defecation. Therefore, further studies are needed in order to confirm whether it is possible to apply FC as a diagnostic tool for the detection of AL after low anterior resection for rectal cancer.
CONCLUSION
In conclusion, this pilot study was the first to evaluate the possible application of the measurement of FC as a diagnostic tool for the detection of AL after low anterior resection for rectal cancer. Thus far, the early signs of AL can only be detected by inflammatory markers in blood tests and/or the findings of physical examinations. However, the examination of FC as an indicator of local inflammation may have the potential to detect the presence of local inflammation due to AL without the influence of other systemic inflammatory complications. This approach, using FC as a marker of local inflammation, may be useful for differentiating local complications from systemic complications.
FUNDING
This study was supported by Koseikan Institutional Research Grant.
CONFLICT OF INTEREST STATEMENT
None declared.
AUTHORS’ CONTRIBUTIONS
M.H. and T.T. designed this study. M.H., T.T., H.S., S.M., H.K. and K.K. treated the patients. Y.S. contributed to the measurement of FC. M.H. and E.S. (specialist in statistics) analyzed the data. M.H. and T.T. interpreted the results and wrote the manuscript.
ETHICS APPROVAL
The medical ethics committee of Saga Medical Center Koseikan reviewed and approved this study design (permission number: 20-06-01-01).
CONSENT TO PARTICIPATE
All patients and their families were informed about the surgical procedure and provided their written consent. Informed broad consent for this study was obtained.
DATA AVAILABILITY
The data sets generated during the study are available from the corresponding author on reasonable request.



