-
PDF
- Split View
-
Views
-
Cite
Cite
Yusuke Yoshikawa, Keiichi Suzuki, Takeo Hashimoto, Kenshi Omagari, Taketo Sasaki, Yusuke Tomita, Akihiko Tamura, Clinical complete response maintained for more than 5 years after aggressive chemoradiotherapy for advanced rectal cancer with necrotizing fasciitis: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 6, June 2023, rjad292, https://doi.org/10.1093/jscr/rjad292
Close - Share Icon Share
Abstract
We report the case of a 65-year-old male diagnosed with advanced rectal cancer associated with necrotizing fasciitis (NF). Since radical surgery, total pelvic exenteration with sacrectomy, was rejected because of detrimental effects on quality of life, chemoradiotherapy (CRT) was chosen as anti-cancer treatment after urgent debridement. Although CRT was paused unintentionally just after delivering the total dose of radiation owing to the relapse of NF, the patient has maintained clinical complete response (cCR) without any distant metastasis for >5 years. Advanced rectal cancer is recognized as an NF risk factor. No definitive treatment strategies have been reported for NF-inducing rectal cancer; however, some reports have demonstrated curative extended surgery. Thus, CRT may be a less-invasive treatment option for NF-inducing rectal cancer, whereas severe adverse effects including re-infection after debridement should be closely monitored.
INTRODUCTION
Necrotizing fasciitis (NF) is a severe and potentially lethal soft tissue infection that is sometimes caused by colorectal cancer [1]. Previous reports have demonstrated curative colorectal cancer resection after stabilizing Fournier’s gangrene (FG), which is a specific form of NF localized around male genital organs; however, there has been no definitive treatment strategy for such patients [2, 3].
Herein, we report the case of a patient with advanced rectal cancer who underwent chemoradiotherapy (CRT) after debridement for rectal cancer-induced NF and eventually maintained clinical complete response (cCR) for more than 5 years.
CASE REPORT
The patient was a 65-year-old man with no previous illnesses. He experienced melena for about 6 months. His oral intake gradually decreased until he could no longer ambulate due to poor nutritional intake, thus prompting consultation at our hospital. The patient also experienced hip pain, and digital rectal examination revealed a solid tumor. Initial laboratory data indicated severe inflammation (white blood cell count of 20 800 cells/μL, platelet count of 5.31 × 105/μL and C-reactive protein level of 15.3 mg/dL). Computed tomography (CT) revealed a progressive rectal tumor infiltrating the prostate and swollen lymph nodes around the distal superior rectal artery (Fig. 1A and B). Fluid and gases were also found in the pelvic cavity and they broadly extended toward the perineal area (Fig. 1C).
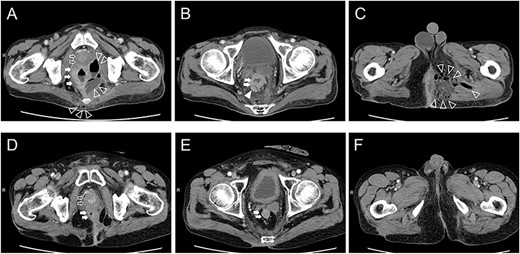
Computed tomography findings upon admission (A–C) and 1 year after chemoradiotherapy (D, E). Prostate level (A, D): The prostate, pelvic cavity and coccyx were invaded by the rectal tumor, fluid and gases (A). The rectal tumor clearly shrank, and only inflammatory scars remained (D). Seminar vesicle level (B, E): Lymph nodes around the superior rectal artery were swollen (B). Swollen lymph nodes disappeared (E). Perineal level (C, F): Fluid and gases extended to the left side of the perineal area (C). Only inflammatory scars remained (F). Open arrow: prostate, closed arrow: rectum, open arrowhead: fluid and gases, closed arrowhead: lymph nodes.
The patient was diagnosed with NF induced by advanced rectal cancer, which was managed by urgent debridement to remove necrotic tissue from the midline of the perineum to the sacral bone based on CT imaging (Fig. 3A) and sigmoid colon stoma creation. Biopsies revealed adenocarcinoma (tub1 > tub2), and the clinical diagnosis was rectal cancer cT4b (prostate) N2aM0 cStageIIIc according to the Union for International Cancer Control Tumor, Node Metastasis Classification of Malignant Tumors, Eighth Edition [4].
After the infection was stabilized, total pelvic exenteration with sacrectomy was planned as a curative treatment; however, this was deferred due to the loss of quality of life with both colostomy and ileal conduit. Therefore, CRT was suggested as an alternative treatment. Chemotherapy (CapOx) consisted of intravenous oxaliplatin administration (130 mg/m2) on day 1 and oral capecitabine (3000 mg/day) on days 1–14, with a cycle interval of 21 days. The total dose of radiotherapy was 67 Gy, which was administered in a fractionated manner (Whole-pelvic irradiation ((45 Gy [1.8 Gy × 25 Fr]) and a radiation boost targeting the primary tumor (22 Gy [2.0 Gy × 11 Fr]).
Radiotherapy was completed and three courses of CapOx were administered at full dose. However, the patient developed fever 2 days after the administration of capecitabine. Although CT revealed no infectious findings, even in the perineal area (Fig. 2A and B), the fever persisted. Five days after CT, magnetic resonance imaging (MRI) was performed to rule out osteomyelitis, and an abscess extension in the perineal area was detected unexpectedly (Fig. 2C–F). Consequently, the patient underwent additional debridement, during which not only the abscess that extended to the right side of the perineum (confirmed on MRI) but also the possible remnant of necrotic tissue around the previous debridement site were removed (Fig. 3B). Subsequently, chemotherapy was discontinued. Eight months after the last day of CRT, the wound was completely closed (Fig. 3). Twelve months after the last day of CRT, clinical complete response (cCR) was confirmed by surveillance CT and colonoscopy (Figs 1D, E and 4). Since then, the surveillance has been continued, with no recurrence noted for >5 years.
![Computed tomography (A and B) and magnetic resonance imaging (C–F) during the relapse of necrotizing fasciitis. Retrospectively, the attenuation in perineal area seemed to be slightly increased, but no obvious fluid or gases could be found on computed tomography (A and B). However, magnetic resonance imaging (T1 fat saturated [C and D] and diffusion weighted [E and F]) clearly revealed abscess formation in the perineal area.](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2023/6/10.1093_jscr_rjad292/1/m_rjad292f2.jpeg?Expires=1772474670&Signature=Z2QX8aV25ufa01RqXQMYkqH0QJjZLegbhe4EfkECGo~jGQ-TpDDiu3fBAzYUO3li7wyfJOID4H3WEe-jFJRcitwKBmEsYFFI0BUNg~e5LSdSkoJR1ZvfI2kxsAaH4iiAyn1hblcoAEEVMl2afXL9O5kCjjn80Fsoz3FFkjmXtArTxUirnMXWNiRyFXfK-0eEawdmLqXWeBELRKv93XAiG8i7zyKOfqQk77nimlhDGkr1Sqw2bf-qcuNZAKdA7AciWMZBh5ZTtSfRg896XkDxwFtFUFujsbRzIERZBZy8NxEP3cguvMx7O2gTVaw7wMLpKyI5sttotGdNmJUAmQO8aQ__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
Computed tomography (A and B) and magnetic resonance imaging (C–F) during the relapse of necrotizing fasciitis. Retrospectively, the attenuation in perineal area seemed to be slightly increased, but no obvious fluid or gases could be found on computed tomography (A and B). However, magnetic resonance imaging (T1 fat saturated [C and D] and diffusion weighted [E and F]) clearly revealed abscess formation in the perineal area.
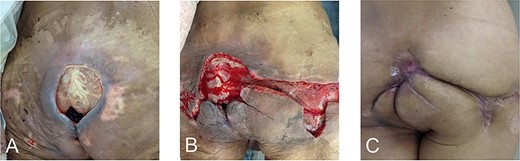
Wounds and scars after debridement. The wound during primary debridement (A) and additional debridement upon the relapse of NF (B), which healed about 9 months later (C).
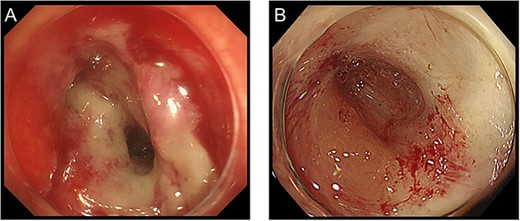
Colonoscopy findings before (A) and at 12 months after CRT (B).
DISCUSSION
NF is a progressive infection in the fascial planes that causes necrosis of the subcutaneous tissue [5]. The most common cause of NF is trauma, but complicated intra-abdominal infections, including colorectal cancer perforation, are also one of the major etiologies [1]. Prompt surgical intervention and appropriate antibiotics are essential for NF treatment [6]. Despite radical treatment, the mortality rate of NF ranges 20–40% [7, 8]. Moreover, the management of colorectal cancer-induced NF is highly complex, and oncological treatment must be planned while controlling the infection.
Bruketa et al. proposed primary tumor resection as an oncological treatment for colorectal cancer-induced FG after stabilizing infection [2, 9]. However, curative surgery for such patients is occasionally accompanied by extensive physical damage or detrimental effects on quality of life. In this case, total pelvic exenteration with sacrectomy was initially planned but deferred by the patient, who accepted CRT instead. An aggressive combination of chemotherapy and radiotherapy, including recent total neoadjuvant therapy, possibly results in a higher cCR rate [10]. Burbach et al. demonstrated that dose escalation above 60 Gy (60–75 Gy) for locally advanced rectal cancer results in high cCR rates and acceptable early toxicity [11]. The combination of radiation and CapOx has also been reported as an enhanced form of induction therapy [12]. Based on these reports, we ultimately chose high-dose radiation (67 Gy) and CapOx despite the potential high risk of adverse effects. Unfortunately, the patient was scheduled for re-debridement for the relapse of NF, which was detected on MRI. MRI, which can provide more accurate delineation of the disease and even identify unexpected sources of infection in some cases, is not widely used owing to the high cost [1, 13]. However, in the present case, MRI should have been scheduled promptly when no findings were detected on CT during the febrile episode, as CRT for advanced rectal cancer can be a risk factor for another NF [14].
Eventually, the patient has achieved more than 5 years of disease-free survival. According to Peterlli et al., cCR after radiation with or without chemotherapy is not a surrogate endpoint for overall survival in rectal cancer [15]. Thus, CRT should be planned as neoadjuvant therapy until accumulating evidence reveals characteristics that allow patients to be observed safely without surgery after CRT. On the other hand, curative CRT for rectal cancer should be further studied, and this case report indicates that aggressive CRT might be beneficial for the treatment of patients with NF-inducing rectal cancer, although strict monitoring is necessary for the early detection of adverse effects, such as relapse of NF.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflicts of interest.
FUNDING
This study was not funded.
DATA AVAILABILITY
The datasets supporting the conclusions of this article are included in this article.
ACKNOWLEDGEMENTS
The authors would like to thank Enago (www.enago.jp) for the English language review.



