-
PDF
- Split View
-
Views
-
Cite
Cite
Abdullah K Alassiri, Fahad M Alshair, Mazin A Fatani, Abdullah H Baghaffar, Aortic root abscess and Brucella endocarditis in a patient with mechanical aortic valve prosthesis: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 6, June 2023, rjad299, https://doi.org/10.1093/jscr/rjad299
Close - Share Icon Share
Abstract
Rare but potentially fatal, brucellosis prosthetic valve endocarditis is a complication of brucellosis caused by Brucella species. The symptoms of brucellosis can be nonspecific, making the diagnosis challenging. Osteoarticular involvement is the most common complication of brucellosis. Mortality from brucellosis is low except for endocarditis and involvement of the central nervous system. The diagnosis is based on laboratory tests and clinical manifestations. Serological tests are preferred, as culture methods can be unreliable. A 59-year-old woman presented with gastrointestinal bleeding, fever, anorexia and malaise. She had a history of aortic valve replacement with a mechanical prosthesis for severe bicuspid aortic stenosis. Investigations revealed a multiloculated aortic root abscess encircling the prosthetic valve. She was diagnosed with brucella endocarditis, treated with antibiotics and underwent cardiac surgery. Her symptoms improved following the surgery. Brucellosis prosthetic valve endocarditis is a rare presentation of this disease.
INTRODUCTION
A zoonotic disease caused by bacteria from the genus Brucella is known as brucellosis. With a global distribution, it is most prevalent in the Mediterranean basin, the Middle East, India, Mexico and Central and South America [1]. Eighty percent (80%) of deaths from brucella are caused by brucella endocarditis, which affects 0.5–2% of all cases [2]. The severe multisystemic illness known as human brucellosis can affect any organ. There could be localized complications, therapeutic failure and relapse. Endocarditis is the most frequent cardiovascular complication of the disease, although cardiovascular complications are generally rare [3]. Cardiac failure typically results in death, especially in those with a late diagnosis [4].
CASE PRESENTATION
A 59-year-old female presented to the emergency room with a 1-week history of lower gastrointestinal bleeding, chronic fever, anorexia and malaise. She had a medical history of aortic stenosis and had undergone aortic valve replacement with a mechanical prosthesis at the age of 49. She also had comorbidities of osteoporosis and heart failure with preserved ejection fraction (HFpEF). An upper gastrointestinal endoscopy revealed no active bleeding lesions. Laboratory workup showed anemia with a hemoglobin concentration of 4.9 g/dL and a supratherapeutic international normalized ratio (INR), which was attributed to the 6 mg dose of warfarin. The patient was treated with packed red blood cells, ferrous sulfate, folate and vitamin B12, and her hemoglobin reached 8.3 g/dL with symptom improvement.
A thorough cardiovascular examination was performed, which revealed normal prosthetic valve clicks and a grade 3 ejection systolic murmur in the right second parasternal space. Further investigations included a transesophageal echocardiogram (TEE), which showed a paravalular anechoic free space around the prosthetic mechanical aortic valve with paravalular leakage and regurgitant flow with dehiscence of the prosthesis causing aortic stenosis (Fig. 1). A computed tomography (CT) scan was also performed to rule out aortic calcification, which revealed a normally seated aortic valve prosthesis with no visualization of the paravalular free space (Fig. 2). During the preoperative workup, blood cultures showed bacterial growth of Brucella melitensis. A multidisciplinary team approach was initiated, and the patient was started on antimicrobial therapy with gentamicin, doxycycline and rifampin. However, due to the patient developing abnormally high levels of Aspartate aminotransferase (AST) and Alanine aminotransferase (ALT), which is a side effect of rifampin, the regimen was changed to ceftriaxone, doxycycline and trimethoprim/sulfamethoxazole.
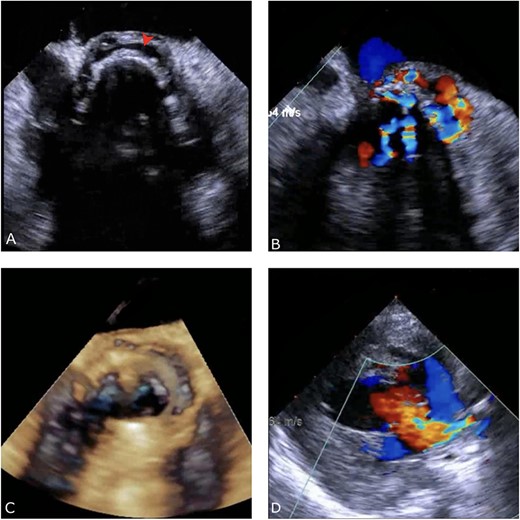
Preoperative TEE: (A) Red arrow pointing at a paravalvular anechoic free space around the prosthetic mechanical aortic valve. (B) Echo image showing paravalvular regurgitation and leakage around the prosthetic mechanical valve. (C) 3D reconstructive echocardiography image showing paravalvular free space around the aortic mechanical valve at 3–9 o’clock with aneurysmal echo free space at the aortomitral curtain protruding into left atrium. (D) Aortic prosthetic mechanical valve dehiscence causing aortic stenosis with moderate paravalvular regurgitation.
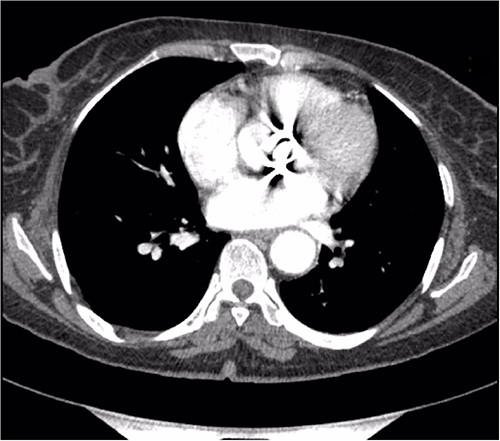
Preoperative CT demonstrating a previous aortic mechanical valve prosthesis.
The patient underwent surgery using a j-aortotomy approach, which allowed for excellent visualization of the prosthetic valve, revealing dehiscence at the right and the non-coronary sinuses and a completely destructured aortic valve annulus. The previous aortic prosthesis was removed, and the annulus was reconstructed using a 1.5 cm wide pericardial patch, with the lower edge of the patch being secured using an interrupted pledgeted proline stitch while the rest of the patch was secured using a continuous running proline suture. The annulus was sized and it was too small for a size 19 valve, therefore it was increased by a nicks procedure, extending the aortotomy incision, which was then patched using another pericardial patch. The valve was sized as a size 19 bioprosthetic valve and secured, and the aortotomy incision was closed in a single layer.
The patient had an uncomplicated postoperative course in the intensive care unit and was then shifted to the surgical step-down unit. However, the patient developed a superficial sternal wound infection, which was managed with the use of a vacuum-assisted closure (VAC) device. Once the wound had healed, it was closed under general anesthesia with an incisional VAC device installation. After 3 months, the patient underwent a follow-up transthoracic echocardiogram (TTE), which showed a normally functioning aortic valve prosthesis with normal global left ventricular function (Fig. 3).
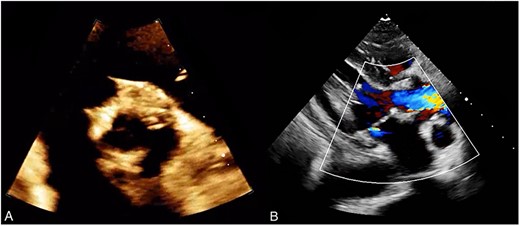
(A) Post operative TTE demonstrating a bioprosthetic valve in proper position. (B) Normal functioning bioprosthetic aortic valve with no signs of stenosis or regurgitation.
DISCUSSION
Brucella endocarditis is a rare complication of brucellosis. Fever, chills, osteoarticular and muscular symptoms are the most prevalent signs of brucellosis. The disease typically takes 2–6 weeks to incubate, but this can occasionally be much longer. This explains why the disease may become clinically evident far from the site of infection. The diagnosis of focal forms of brucellosis can be particularly challenging because, even when taken into consideration by the doctor as a possible cause of the symptoms, the yield of cultures from blood and non-blood samples is low [5, 6].
It is well known that Brucella infection can cause severe valvular damage that needs to be repaired surgically [4]. A study by Colmenero et al. [7], reported the development of endocarditis in only 1.13% patients (6 out of 530 patients) with brucellosis. A similar study also concluded the rarity of development of brucella endocarditis [8]. Peery and Belter [9] found endocarditis in 80% of brucellosis patients who died, highlighting the significance of including the disease in differential diagnosis and seeking a prompt diagnosis.
Brucella endocarditis is frequently associated with a much higher proportion of negative blood cultures than endocarditis caused by other bacteria, and in many series, it is part of the pool of cases of pathogen-induced endocarditis with negative blood cultures due to the slow growth rate of Brucella spp. and their requirement of a suitable culture medium [10]. It has also been reported that transesophageal echocardiography could have revealed more details about the extent of valvular damage, the presence of ring or myocardial abscesses and the development of aneurysms [11]. So, including transesophageal echocardiography in the follow-up of brucellosis could help counter even the barest risk of brucella endocarditis. According to reports, the only way to get rid of an infection with a pathogen like Brucella spp., is through a combination of medical and surgical treatments [9, 12]. We present a case of brucellosis in a female who had undergone aortic valve replacement with a mechanical prosthesis for severe aortic stenosis of bicuspid aortic valve. She developed brucella endocarditis, which is a rare complication of the infection. She was started on antibiotic therapy but had to undergo surgical replacement of prosthetic valves. So even the risk of brucella endocarditis in brucellosis is small, yet it should be taken into consideration when defining differential diagnosis for such case presentation.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No funding received.
DATA AVAILABILITY
All data are available in the article.
CONSENT FOR PUBLICATION
All authors reviewed and agreed on the final version of the manuscript.
References
- aortic valve stenosis
- heart valve prosthesis
- prosthetic valve endocarditis
- anorexia nervosa
- endocarditis
- cardiac surgery procedures
- aortic valve replacement
- brucellosis
- fatigue
- fever
- abscess
- brucella
- surgical procedures, operative
- diagnosis
- loss of appetite
- supraaortic valve area
- prostheses
- aortic valve prosthesis



