-
PDF
- Split View
-
Views
-
Cite
Cite
Pir Muneeb Rehman, Fariha Sahrish, Sahar Iqbal, Tuba Tariq, Muhammad Shoaib, Sehrish Rubab, Muhamad Imran, Mehwish Niazi, Saima Irum, Muhammad Aqeel, Rare serosal cystic gastric gastrointestinal stromal tumor with extensive intestinal metaplasia in an adherent gastric mucosa; a case report in a 65-year-old male, Journal of Surgical Case Reports, Volume 2023, Issue 5, May 2023, rjad241, https://doi.org/10.1093/jscr/rjad241
Close - Share Icon Share
Abstract
Gastrointestinal (GI) intestinal stromal tumors account for 60% of mesenchymal GI tract tumors commonly located in the stomach and small intestine, predominantly solid tumors that rarely undergo cystic degeneration. A 65-year-old patient with increasing upper abdominal swelling and a computed tomography scan abdomen showed a large unilocular 17 × 16 × 15 cm lesion. A colossal cystic swelling in the lesser omentum, anterior to the stomach, was found upon exploration. Histopathological examination showed a spindle cell tumor turned out to be CD117 positive and S100 negative on immunostains. The tumor was moderate risk gastric gastrointestinal intestinal stromal tumor (GIST) based on the site; Stomach, Size >10 cm; Mitosis <5/5 mm2 according to risk assessment of GIST, 2006. GISTs are predominantly solid tumors and rarely undergo cystic transformation. The primary differential diagnoses of spindle cell neoplasm are GISTs, Leiomyoma, Leiomyosarcoma and Schwannoma. These spindle cell neoplasms are differentiated by applying a panel of Immunohistochemical stains, CD117, SMA and S100.
INTRODUCTION
Gastrointestinal intestinal stromal tumors (GISTs) are the most common mesenchymal tumor of the gastrointestinal (GI) tract and account for 60% of mesenchymal GI tumors. They can occur anywhere in the GI tract but are most commonly located in the stomach (60%) and small intestine [1]. They originate from the cells of the Cajal, known as pacemaker cells that express receptor tyrosine kinase (KIT) kinase (Tyrosine Kinase Inhibitors), responsible for GI tract motility. The activating KIT mutations are present in 85–95% of GIST; the rest of KIT-negative tumors contain platelet-derived growth factor receptor alpha mutations [2, 3]. Currently, the proper identification of GISTs is crucial due to the targeted treatment regimens available. Most GISTs present at age above 50 years with no known risk factors. The clinical presentation of GISTs is highly variable, from asymptomatic or can present as abdominal pain, obstruction and bleeding. GISTs usually form present as a solid mass [4]. The size of these tumors varies from a few millimeters to several centimeters in diameter. However, they can rarely undergo cystic change with degeneration and calcification. Cystic GISTs are unusually large with cystic and solid areas. GISTs >10 cm in diameter are labeled as giant GISTs. The most frequently reported sites are the stomach, pancreas, intestine and omentum. Hence cystic GISTs are challenging to differentiate from cystic tumors in the areas mentioned above [5, 6].
Here, we report a case of a gastric GIST with a predominant cystic change and extensive intestinal metaplasia (IM) in an adherent gastric sleeve. The cystic GIST was en-bloc excised, without regional lymphadenectomy.
CASE REPORT
A 65-year-old patient with increasing upper abdominal swelling and pain was admitted to the surgical department. The patient had no history of fever, vomiting or melena. The patient underwent craniotomy for extradural hematoma due to a fall. No previous or recent history of trauma to the abdomen was given. On examination, his vitals were normal, and no weight loss was observed. A large, tense and slightly mobile mass in the left upper abdominal quadrant was noted. The rest of the physical examination and the blood investigations were unremarkable. A large unilocular cyst was pointed out in the ultrasound abdomen. The computed tomography (CT) scan abdomen showed a large unilocular 17 × 16 × 15 cm cystic lesion (Fig. 1A and B), closely abutting the anterior gastric wall forming a claw around it. The cyst has a maximum wall thickness of 1.4 cm with no internal solid or papillary areas. However, no gastric obstruction was seen, and the substantial cyst was compressing the pancreatic body. The upper GI endoscopy showed normal gastric mucosa.
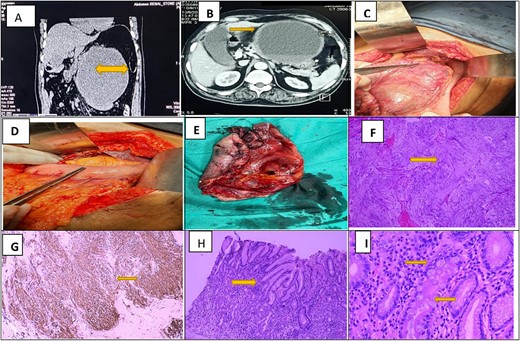
Radiological (CT Scan) and Histopathological features of GI stromal tumor. (A) and (B) Sagital and axial slices show a colossal hypo intense cyst measuring 17 × 16 × 15 cm closely abutting the anterior gastric wall forming a claw around it. The cyst has a maximum wall thickness of 1.4 cm with no internal solid or papillary areas. (C) Intra-operative images of massive cystic swelling adherent to the lesser curvature of stomach approaching lesser omentum and gastrocolic ligament. (D) After cyst resection, reverse sleeve gastrectomy is done in a double-layered fashion. (E) Postoperative image shows a cyst resection specimen with a smooth external surface and internal hemorrhagic surface. (F) Photomicrograph of H&E stain shows neoplastic spindle cells arranged whorls and palisading of nuclei noted. (G) Strong and diffuse cytoplasmic staining of CD 117 in tumor cells. (H) and (I) Photomicrograph of H&E stain shows low and high-power view of adjacent gastric mucosa with extensive chronic changes (Crock screwing, budding, branching and foveolar hyperplasia) and IM (highlighted by yellow arrows).
After a complete pre-assessment, this patient was discussed in a review board with the radiologist, and an exploratory laparotomy was planned. After general anesthesia, the patient was explored through an upper midline incision. A colossal cystic swelling in the lesser omentum, anterior to the stomach, was found upon exploration. The cyst adhered to the lower curvature of the stomach (Fig. 1C and D), containing 1700 ml brownish color fluid. Since, the cystic mass was not adherent to adjacent organs, an en-bloc excision with reverse sleeve gastrectomy was done. The stomach was primarily closed in a double-layered fashion and pyloroplasty was done. Grossly identified as an already incised irregular tissue mass with a smooth outer surface and the internal hemorrhagic surface. Adherent gastric mucosa was identified and sectioned along with cystic wall tissue. Histopathological examination showed a spindle cell tumor turned out to be CD117 positive and S100 negative on immunostains. It was graded as moderate risk gastric GIST based on the site; Stomach, Size>10 cm; Mitosis <5/5 mm2 according to Risk assessment of GIST, 2006 by Miettinen and Lasota. Interestingly, the adjacent gastric sleeve shows extensive IM (Fig. 1E and F). Postoperative recovery was uneventful. The patient was referred to the relevant oncologist for imatinib therapy. Since, there was extensive IM in the adherent gastric sleeve was noted microscopically, the patient was put on active endoscopic surveillance.
DISCUSSION
GISTs are predominantly solid tumors, rarely undergoing cystic transformation and present as huge abdominal masses. Huge exophytic cystic GISTs are often confused with cystic neoplasms of the liver, pancreas and omentum; hence challenging to diagnose and operate. The transcutaneous ultrasound-guided Fine Needle Aspiration Cytology or biopsy of cystic tumors carries the risk of rupture, thus resulting in intra-abdominal spillage [7, 8]. Usually, previously treated epithelial and mesenchymal tumors undergo a cystic change. Our index case did not receive treatment, so the cystic change etiology is unclear. The primary differential diagnoses of spindle cell neoplasm are GISTs, Leiomyoma, Leiomyosarcoma and Schwannoma. These spindle cell neoplasms are differentiated by applying a panel of Immunohistochemical stains, CD117, SMA and S100 [9, 10].
According to the literature, the demographic data regarding age of presentation, sex and specific location is the same as reported by other authors [1, 3, 5, 6, 8]. In the index case, the tumor adhered to the serosal surface of lesser curvature, as reported by other authors [1, 3]. However, other sites like the posterior gastric wall, retro gastric region and entire stomach wall were also reported [5, 6, 8]. Our index case showed gigantic cystic GISTs of 17 cm, similarly penned by other authors; to date, a massive cystic GIST of 42 cm was also reported [6, 8]. The majority of tumors, including our case, were PT4, non-invasive tumors similarly written by other authors [1, 5, 6]; however, the adjacent organ, like the liver, pancreas and spleen involvement, was also penned by other authors [3, 8].
In the index case, the adherent gastric sleeve shows foveolar hyperplasia, corkscrewing, cystically dilated glands and extensive IM similarly reported by Kamel Adwan A et al., Zamecnik M et al. and Kinsinger LA et al. As IM is a pre-malignant condition, rarely synchronous GIST and Adenocarcinoma is reported [11]. The patient was advised follow-up endoscopic examinations by a gastroenterologist as per guidelines. [11–14].
CONCLUSION
We encountered a rare case of a gastric GIST with predominant cystic formation, suggesting cystic GISTs should keep in mind while detecting intra-abdominal cystic lesions. In addition, since the adjacent gastric sleeve showed extensive IM, which was not seen in the endoscopic examination, surveillance with endoscopic ultrasound is highly recommended.
ACKNOWLEDGEMENTS
I acknowledge Dr. Pir Muneeb Rehman’s diligent efforts for this patient and helping with the case report.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
There is no funding to be disclosed.
References
Babaria SS, Dayal A, Thakkar HP, Joshi DS..



