-
PDF
- Split View
-
Views
-
Cite
Cite
Giedrė Žulpaitė, Rūta Žulpaitė, Alvydas Vėželis, Scrotal squamous cell carcinoma: a case report, Journal of Surgical Case Reports, Volume 2023, Issue 3, March 2023, rjad128, https://doi.org/10.1093/jscr/rjad128
Close - Share Icon Share
Abstract
Scrotal squamous cell carcinoma (SCC) is a rare condition that typically manifests in the sixth decade of life and usually presents as a painless, solitary nodule that slowly increases in size, ulcerates, and gets infected. The diagnosis is often delayed, as the majority of patients tend to avoid seeking medical help due to embarrassment. We present a 62-year-old male with a massive 8 cm ulcerating painless tumor in the scrotum. A patient underwent scrotal extirpation with bilateral orchofuniculectomy and diagnostic bilateral inguinal lymphadenectomy. Histopathology revealed a well-differentiated SCC of the scrotal skin. Ignorance, lack of self-awareness, knowledge about risk factors and aggravated access to healthcare facilities remain important reasons for late diagnosis.
INTRODUCTION
Scrotal squamous cell carcinoma (SCC) is a rare condition with an incidence of 0.3–1.5 cases per 1 000 000 males [1–4]. It presents as a painless, solitary nodule that slowly increases in size, ulcerates, gets infected. The diagnosis is often delayed, as patients tend to avoid seeking medical help due to embarrassment [2]. We present a rare case of massive ulcerating scrotal SCC observed by the patient for two years.
CASE PRESENTATION
A 62-year-old male with no comorbidities was admitted to the urological oncology department with an 8 cm ulcerating painless tumor in the scrotum (Fig. 1A). He has been observing the formation for two years but has not sought medical help. β-Human Chorionic Gonadotropin concentration was slightly elevated (2.31 mIU/mL). Other laboratory tests were within normal limits.
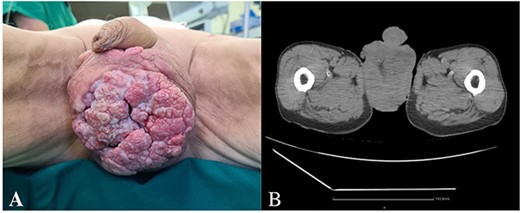
(A) Ulcerating painless tumor in the scrotum, (B) heterogenous tumor mass in the projection of scrotum (CT).
Radiological findings
A whole-body computed tomography (CT) scan showed a heterogenous tumor mass in the scrotum, involving the testicles, surrounded by free fluid (Fig. 1b). Bilaterally enlarged inguinal lymph nodes (l/n), but no metastases in bones or visceral organs were observed.
Treatment
The patient underwent scrotal extirpation with bilateral orchofuniculectomy and diagnostic bilateral inguinal lymphadenectomy. Both spermatic cords were divided by an incision along the inguinal canals. The funiculus spermaticus was clamped proximally, dissected distally, cut below the clamping site (Fig. 2A). It was impossible to separate testicles from the surrounding tissues of the scrotum due to the overgrowth of pathological masses and abscess formation. Within the boundaries of the healthy tissue, the skin of the scrotum was circularly cut (Fig. 2B). The scrotum with testicles and spermatic cords was removed in one specimen (Fig. 3A). No overgrowth of the pathological masses into the urethra was observed. The enlarged superficial inguinal lymph nodes (3 cm) were removed bilaterally for diagnostics. The perineal area was drained. The incisions were closed (Fig. 3B).
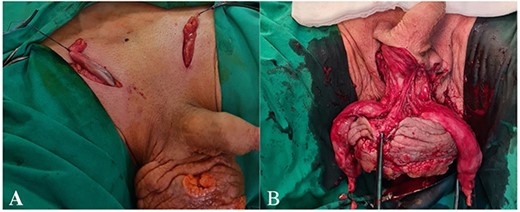
(A) Funiculus spermaticus cut below the clamping site, (B) circularly cut in the skin of the scrotum within the boundaries of the healthy tissue.
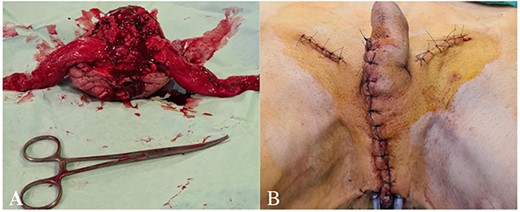
(A) The scrotum with testicles and spermatic cords removed in one specimen, (B) the perineal area drained with two drainage tubes.
In the microbiological specimen, the growth of Streptococcus pyogenes, Proteus penneri, Prevotella ivia, Morganella morganii was detected. The antibacterial therapy was prescribed accordingly.
Pathological findings
Grossly, the scrotum was 13 cm × 12 cm × 8 cm, containing one 3.5 cm × 2.5 cm × 2.5 cm testicle, 1.5 cm × 0.5 cm × 0.5 cm epididymis, 6 cm × 2.5 cm spermatic cord, and another 3.5 cm × 2 cm × 2 cm testicle, 2 cm × 1 cm × 0.5 cm epididymis, 9 cm × 2.5 cm spermatic cord. A whitish, fragile 10 cm × 9 cm × 6 cm tumor was visible in the skin and soft tissues of the scrotum. Macroscopically, the tumor was located 0.8 cm from the skin resection margin.
The final pathological diagnosis was well-differentiated (G1) SCC of the scrotal skin, TNM: pT3 (10 cm tumor), N0 (0/3 l/m), LVI-0 (lymphovascular invasion not found), 5-mm length spread at the edge of skin resection, differentiated intraepithelial neoplasia of the scrotum (PeIN analog), reactive lymphadenopathy.
Follow-up
After 1 month, no clinical relapse was observed. Early radiological follow-up was planned with a full-body CT scan and pelvic MRI 3 months after the surgery.
DISCUSSION
Tumors of the scrotum can emerge from every layer [5], but SCC is the most common (one-third of cases). [3]. Other scrotal tumors are extramammary Paget’s disease, sarcoma, basal cell carcinoma, melanoma or metastasis [2, 4]. They might appear as papules, nodules, localized edema and need to be differentiated from inflammation, lymphangioma, hemangioma [6].
Historically, SCC has been identified as an occupational disease [4]. Nowadays, it is mostly associated with poor hygiene, chronic irritation, radiation history, human papillomaviruses [5, 7], previous radiotherapy treatment [2]. Nevertheless, none of these risk factors were reported by our patient.
Patients, as well as our patient, delay for a 2.2–3.3 years seeking medical help due to bashfulness and ignorance [5, 8–10]. Nevertheless, cases of rapid scrotal SCC progression with ulcerations and infection have been previously reported [5, 7]. In the present case, abscess formation in the tumor was also observed.
Scrotal SCC rarely affects the testicles, epididymis, spermatic cord. Nevertheless, as in our case, inflammatory infiltration of the scrotum might mimic the testicle’s involvement. An inflammatory or neoplastic enlargement of inguinal lymph nodes bilaterally may also appear and later spread to the pelvic lymph nodes [11]. The spread to the superficial and deep inguinal canal bilaterally or unilaterally, iliac, paraaortic lymph nodes para-aortic have also been reported [5]. Interestingly, tumors that do not affect the median raphe have a low likelihood of spreading to the opposite inguinal area [11].
Due to the rarity of scrotal SCC, the evidence regarding the management of this condition is scarce [2]. Surgical removal with at least 2–3 cm wide clean margins is the most recommended way of treatment [10]. In our case, scrotal extirpation with bilateral orchofuniculectomy and diagnostic bilateral inguinal lymphadenectomy was performed. For comorbid patients who cannot undergo surgery, alternative options such as CO2 laser for invasive SCC and imiquimod, photodynamic therapy and 5-fluorouracil for SCC in situ may be used [12]. Removal and evaluation of the sentinel lymph node via biopsy and lymphadenectomy of ilioinguinal, inguinal l/m are commonly recommended for individuals with suspected metastases to l/m [1]. In the case of a massive tumor, neoadjuvant radiotherapy and chemotherapy are used to reduce the size of the tumor and achieve complete removal [13]. There are data in the literature showing a better survival of scrotal SCC patients who received adjuvant RT and four cycles of chemotherapy (cisplatin, bleomycin or methotrexate) [14]. The prognosis depends on the histologic grade, surgical margin, infiltration: patients with first-stage scrotal SCC have a good chance of 5-year survival (75%), whereas those with more advanced (C and D stages) have a poor prognosis: 4 and 8 months of survival, respectively [2, 15], 25% of patients with inguinal l/n metastases survives 5 years [5] and no one survives 5 years with iliac nodal metastases [8].
CONCLUSION
Scrotal SCC is a rare but consequential health problem. Due to patients’ shyness and ignorance, the diagnosis is often delayed. Self-awareness of patients, knowledge about risk factors, easy access to healthcare facilities are crucial to reducing the number of advanced diseases at the time of diagnosis. Because of the rarity of the disease, there are still no definite scrotal SCC treatment guidelines. Radical surgery is currently considered the primary principal method of treatment.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
PATIENT CONSENT
We received written informed consent from the patient to publish the case and pictures.
AUTHORS' CONTRIBUTIONS
All authors contributed equally to this work. All authors have read and approved the final manuscript.



