-
PDF
- Split View
-
Views
-
Cite
Cite
Vladimir Yu Struchkov, Stanislav V Berelavichus, Mihail V Dvuhzhilov, Evgenij A Akhtanin, Andrej G Kriger, Complex treatment of multiple high-output enterocutaneous fistulas (gastric, jejunum, transversum) after bariatric surgery, Journal of Surgical Case Reports, Volume 2023, Issue 3, March 2023, rjad096, https://doi.org/10.1093/jscr/rjad096
Close - Share Icon Share
Abstract
Multiply high-output enterocutaneous fistulas (ECF) is a tragic postoperative complication. This report describes complex treatment of patient with multiple enterocutaneous fistulas after bariatric surgery, including a comprehensive preoperative preparation for 3 months (sepsis control, nutritional support and wound care) and reconstructive surgery (laparotomy, distal gastrectomy, resection of the small bowel with fistulas, Roux-gastrojejunostomy, transversostomy).
INTRODUCTION
The development of a postoperative enterocutaneous fistula is always a problem both for the patient and for the doctor. ECF’s are one of the most severe and hard to treat complications in the abdominal surgery. Especially challenging is the treatment of multiple fistulas of the gastrointestinal tract. Difficulties in the planning of the management approach, high recurrence rate, mortality rate of more than 60% determine the significance and importance of the problem.
A functioning ECF maintain the abdomen and wound inflammation, lead to electrolyte imbalance, severe dehydration, malnutrition and sepsis and the belated surgical therapy result in the worsening of the patient’s condition.
CASE REPORT
A 37-year-old man was admitted to the abdominal surgery department with pain, open postoperative wound in the left subcostal area and multiplies enterocutaneous fistulas with daily output of up to 2000 ml of gastric and enteric contents.
His medical history included high-degree obesity (BMI 48 kg/m2) with many unsuccessful efforts of weight loss. His surgical history included bariatric surgery—distal gastrectomy, Roux-en-Y gastric bypass with Braun anastomosis and Shalimov plug (staple line on proximal intestine) (Fig. 1).
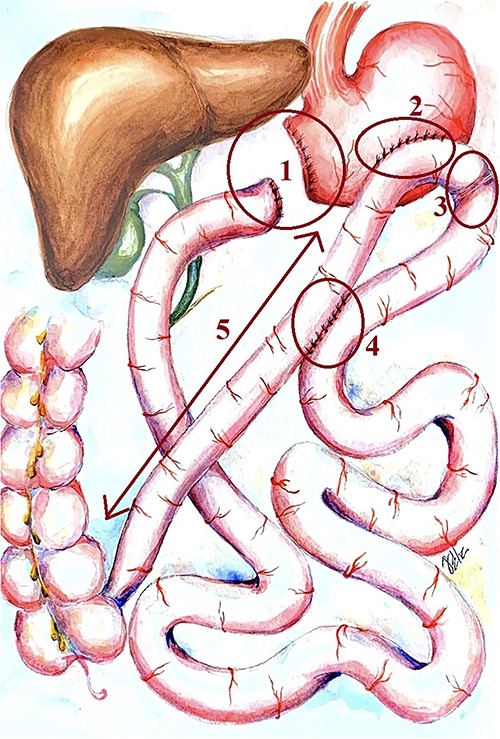
Scheme of the primary bariatric surgery. 1: gastric stump and duodenic stump, 2: gastroenteroanastomosis bypass, 3: Shalimov’s plug (staple line on proximal intestine), 4: Braun anastomosis and 5: 1 m length ileal loop between the gastroenteroanastomosis and the ileocecal angle.
On the 3 and 17 postoperative days because of adhesive small bowel obstruction, an emergency surgery were performed including relaparotomy, adhesiolysis, nasointestinal intubation.
On the 21 postoperative day (4 days after the last relaparotomy), the gastroenteroanastomosic leakage with peritonitis occurred. Surgical wound dehiscence was performed. Patient was started total parenteral nutrition and fistuloclysis. The daily fistula output was about 2000 ml of gastric and enteric contents.
Under the complex non-surgical treatment patient’s condition stabilized, but on the 61 p/o day patient underwent surgical fistula closure (suturing a fistula in a wound). On the three postoperative day fever of 38.7°C started, and bowel content appeared in the wound. Surgical wound dehiscence was performed, fistula output increased to 2000 ml per day.
To lower the fistula, output on the 71 postoperative day performed emergency relaparotomy in the left subcostal area, bypass gastroenterostomy. The postoperative period was followed with septic shock, multiple enteroatmospheric fistulas, output of bowel content up to 2500 ml per day and abdominal wall phlegmon in the left subcostal area.
On the 86 day from the initial bariatric surgery, an emergency relaparatomy was performed. Collateral enteroenteric anasthomosis was formed between the afferent enteric loop and the distal ileum to disconnect the fistula from functioning GI tract. This surgery had no positive effect—the fistula output did not decrease.
After the unsuccessful surgical treatment, patient was transferred to our clinic. Weight loss during the last 5 months was 55 kg.
Status localis: patient had an abdominal wall wound of irregular shape 15 × 10 cm with rough edges in epigastrium. Wound edges were covered with fresh granulation tissue. Wound bottom was formed by small bowel loops. In the lower left corner of the wound, an enterocutaneous fistula 2.5 cm diameter was seen. In the right upper corner, an enterocutaneous fistula 1 cm in diameter, which contains Kehr’s T-tube with no output—was removed during the wound revision. In the left upper corner of the wound, gastroenteric anastomosis leakage with the enteral feeding tube inside could be visualized. Close to this leakage, two small bowel lumens were seen filled with enteric contents with bile. The wound lasts up to the left subcostal area to the anterior axillary line forming a recess anterior abdominal wall. This recess was drained with a drainage tube through a counterincision. Skin in the left subcostal wound area was inflamed, macerated, hyperemic. Small bowel contents were constantly actively aspirated. Fistulas total output reached 1500 ml per day.
Laboratory assessment shown average anemia (Hb 87 g/L), hypoproteinemia (total protein 55 g/L), hypoalbuminemia (albumin 32 g/L), hypokalemia (K 2.9 mmol/L) and hyperlactataemia (lactate 2.3 mmol/L). Coagulation profile shown elevated fibrinogen level (5.1 g/L).
A CT of the chest, abdomen and small pelvis showed pulmonary embolism with no fresh focuses of infarct pneumonia, but revealed free fluid in the left pleural cavity, and atelectasis of the basal segment of the left lung, great defect of abdominal wall with the destruction of VIII-X ribs on the left side, multiple enterocutaneous fistulas, gastroatmospheric fistula formed by gastroenteric anastomosis leakage (Fig. 2), thrombosis of the left common femoral vein, hepatomegaly, liver steatosis, free liquid in the abdomen and small pelvis.
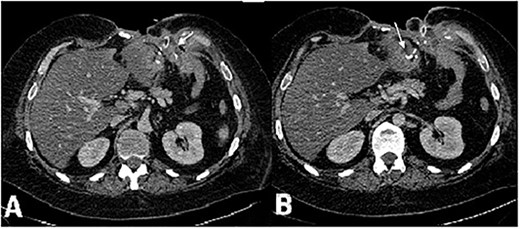
CT scan of the abdomen, axial view, venous phase. (А) Vast defect of the abdominal wall, distal gastric stump and (B) gastroenteroanastomosis leakage. Fistula opening in the wound in the left subcostal area.
Fistulography with contrast was performed via sequential catheterization of all visually accessible fistulas (Fig. 3). Afferent and efferent small bowel loops, gastroenteric leakage, and previously formed enteroenteroanastomosis were identified.
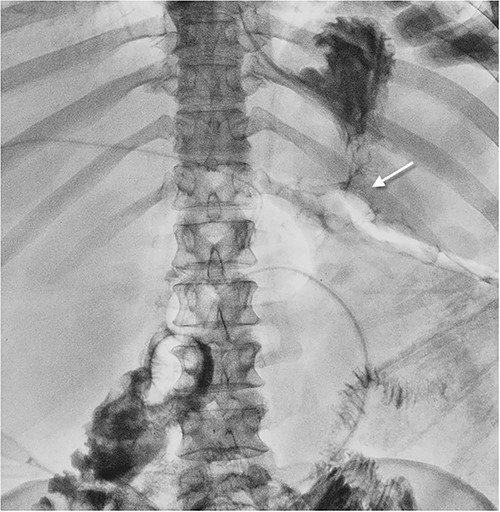
Fistulogram showing gastroenteroanastomosis leakage. The contrast spilling from the gastroenteroanastomosis to the abdominal wall wound.
The first step was to stabilize patient via intensive care management that included electrolyte imbalance correction, sepsis control, nutritional support (TPN, fistuloclysis), therapy of the thromboembolic complications and wound treatment.
Wound management included active aspiration of gastric and bowel contents and skin protection (Fig. 4). Gastric contents were aspirated actively via the nasogastric tube.
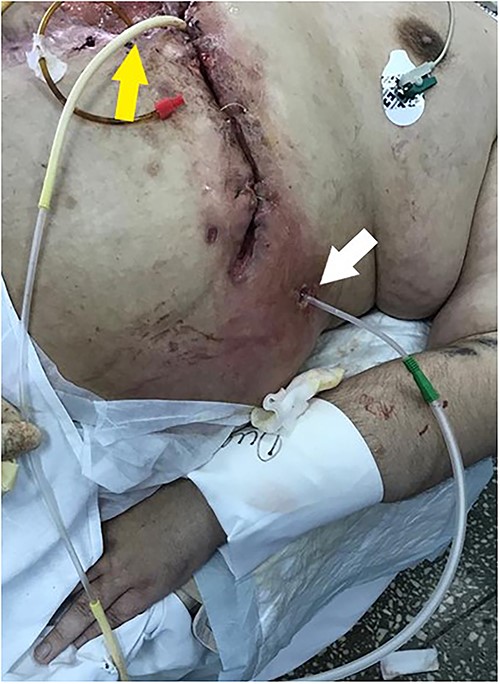
The subcutaneous wound is closed, drainage for the active aspiration is set in the fistula region. The Foley tube for the “fistuloclysis” is intubated into the efferent bowel loop.
After 3 months of complex non-operative management reconstructive surgery was performed: laparotomy, distal gastrectomy, resection of the small bowel with fistulas, Roux-gastrojejunostomy, transversostomy.
The midline laparotomy was performed. Revision of abdomen revealed the flat bowel loops adhesions in the lower part of it that could be easily separated. Three entero-enteric anastomoses formed rings from the small bowel in the upper and middle parts and 5 cm apart the ileocecal angle (Fig. 5). The upper ‘ring’ developed two enterocutaneous fistulas. In the upper region of the laparotomy incision, two separate enterocutaneous fistulas were fixed to the abdominal wall forming a double fistula: the gastric one (leakage of gastroenteric anastomosis) and the transverse colon fistula. Stomach lied under the transverse colon. The antral part was intact (transected after the previously made surgery). Colon was transected through the fistula to allow the gastric stump mobilization. The gastric fistula began from the leakage. Gastroenterostomy formed upon the short loop—less than 20 cm apart the duodenojejunal junction.
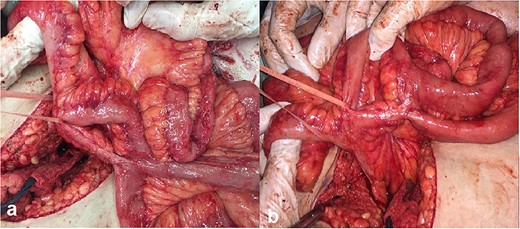
Intraoperative photos: (a) enteroenteroanastomosis in the medium part of the small bowel and (b) two enteroenteroanastomosis 5 cm apart the ileocecal angle.
The bowel was separated from the gastric stump, distal gastrectomy was performed and enteric fistulas resected. Ileum with fistula-forming loops excision was resected. The distal ‘ring’ near the ileocecal angle was not separated. The Roux-en-Y gastric bypass was formed. The distal stump of the transverse colon was closed, and the proximal one formed on the abdominal wall an end colostomy a in the right subcostal area (Fig. 6).
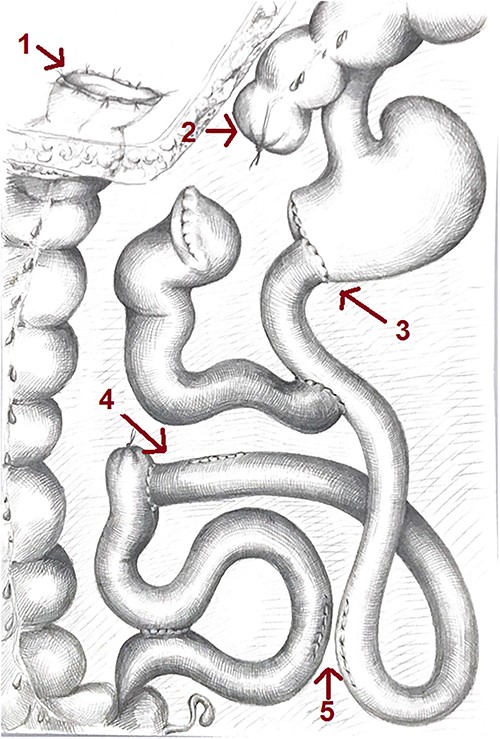
Scheme of the gastrointestinal tract after the reconstructive surgery: 1—colostomy, 2—distal stump of the transverse colon closed, 3—end-to-end gastrojejunoanastomosis, 4—resected enteroenteroanastomosis with the part of the bowel and 5—resected enteroenteroanasthomosis.
Postoperative therapy included total parenteral nutrition for 5 days, infusion, secretion suppression, thromboembolic prevention, daily wound dressing. Postoperatively patient had an uneventful recovery. The wound healed and sutures were removed 15 days post-surgically. Patient was discharged home on the 21 day in good clinical condition.
After 1 year patient underwent laparotomy, adhesiolysis, colostomy closure, transversodescendostomy. No complications were found during the post-surgery period, and the patient was discharged home on the 9 day.
Patient’s quality of life after both surgical procedures was satisfactory.
DISCUSSION
Laparoscopic sleeve gastrectomy and laparoscopic Roux-en-Y gastric bypass account for more than 80% of bariatric surgery procedures [1].
Before the publication of two multi-central studies in 2015 and 2018 SLEEVEPASS (5 year study from March 2008 to June 2010) and SM-BOSS (5 year study from January 2007 to November 2011) the laparoscopic Roux-en-Y gastric bypass operation served for the ‘gold standard’ for its high efficiency in metabolic disorders correction (hyperglycemia, dyslipidemia) and constant body weight loss. However, many authors noted frequent complications, higher risk of anastomotic leakage and complexity of the procedure [1–3].
Five years of intense application of laparoscopic Roux-en-Y gastric bypass no substantial advantages of this technique were revealed [2, 3]. After the publication of the studies mentioned above, sleeve gastrectomy rapidly took the first place in the bariatric surgery. Recently, the sleeve gastrectomy is considered a preferable variant of the morbid obesity surgery for its easiness and lower rate of postoperative complications [1].
The given case demonstrates the incorrect selection of the bariatric surgery approach. The operation chosen included three procedures: partial gastrectomy, gastric bypass and biliopancreatic diversion with duodenal switch. This combination of surgery procedures can be assessed as excessive. Too extensive operation and using of outdated techniques could have been the key reasons for all postoperative complications.
We consider early reconstructive surgery for the one more tactic error. It can be performed only if the inflammatory infiltration of the bowel is absent or minimal. Proceeding from the Russian and foreign authors’ experience optimal time for the reconstructive surgery is between 3 and 8 months after the enterocutaneous fistula formation [4–6]. Untimely operation naturally come to failure and worsen the patient’s condition.
Later multiple attempts to decrease the gastric fistula output via various reconstructive operations worsened the situation and lead to the formation of new bowel fistulas, that increased the gastric and bowel contents loss. An error was made during the ‘backup’ gastroenteric bypass formation, and instead of it the transversogastroanasthomosis was formed. The incorrect choice of colon for the anasthomosis formation was due to the difficulty of the right tissue identification during the surgery of inflammatory infiltrated organs. Moreover, the very idea of the ‘backup’ bypass formation was extremely doubtful and more like an act of despair of the surgeons in a critical situation.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
CONSENT
The patient consented to him case to be published in the scientific literature.
DATA AVAILABILITY
This manuscript presents a case report and therefore the data is not available publicly or upon request to protect the privacy and identity of the patient.



