-
PDF
- Split View
-
Views
-
Cite
Cite
Zhengchao Xu, Ashish Vaska, Ymer Bushati, Angus Alexander, Winston Liauw, Nima Ahmadi, Ruwanthi Wijayawardana, David Morris, Cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy for highly mucinous transverse colon adenocarcinoma in a paediatric patient: a multidisciplinary approach, Journal of Surgical Case Reports, Volume 2025, Issue 10, October 2025, rjaf743, https://doi.org/10.1093/jscr/rjaf743
Close - Share Icon Share
Abstract
We report the first known case of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) performed in a 13-year-old paediatric patient for highly mucinous colorectal adenocarcinoma with peritoneal metastases. This complex procedure was made possible through close collaboration between two tertiary centres, overcoming significant logistical and clinical challenges. The case underscores the importance of flexible multidisciplinary planning in rare paediatric oncology scenarios and demonstrates CRS-HIPEC as a safe and viable treatment option in selected patient with peritoneal malignancies.
Introduction
Mucinous colorectal adenocarcinoma (MCA), defined by >50% extracellular mucin, accounts for 10%–20% of colorectal cancers [1]. It tends to affect the proximal colon, presents at a more advanced stage, shows greater propensity for peritoneal spread, and carries a worse prognosis than non-mucinous variants [1–3].
MCA mainly affects older adults, with an incidence of 7.36 per 100 000 in those aged ≥70 [2]. Paediatric cases are extremely rare and typically linked to genetic syndromes such as Lynch syndrome, with an incidence of 0.12 per 1 000 000 in children aged 0 to 14 and 1.78 per, 000 000 in those aged 15 to 19 [2, 4]. Its rarity often leads to delayed diagnosis and limited treatment options, contributing to poor outcomes.
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) are not established treatments in children and are mainly described in case reports involving paediatric sarcomas and carcinomas. To our knowledge, this is the first reported case of CRS-HIPEC performed in a paediatric patient with peritoneal metastasis from a mucinous colonic primary.
Case presentation
A 13-year-old male with a family history of colorectal cancer presented in 2022 with abdominal pain and vomiting to a tertiary paediatric hospital, initially suspected to be appendicitis. At laparoscopy, a transverse colon mass with pus in the pelvis and paracolic gutter was identified and biopsies taken. Further inpatient evaluation and histopathology confirmed a transmural proximal transverse colon malignancy, and underwent an uncomplicated extended right hemicolectomy with ileocolic anastomosis. Final histopathology confirmed an R0 pT4bN2bM0 high-grade signet ring adenocarcinoma, with extension into greater omentum and 14/97 involved lymph nodes. Genetic analysis revealed high mutational burden, microsatellite instability, mismatch repair (MMR) deficiency, ARIDA1 loss, and BRCA2 mutation.
He received adjuvant FOLFOX and remained well on 3-monthly follow-ups. A positron emission tomography scan in September 2023 showed no recurrence. However, by April 2024, he presented with systemic symptoms and imaging revealed disease recurrence with extensive peritoneal involvement, ascites, pleural effusion, a 10 × 9.7 × 15.4 cm pelvic mass, and right mid-ureteric obstruction requiring stenting (Fig. 1).
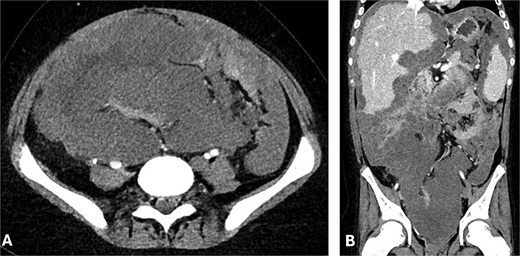
(A) Axial and (B) coronal CT abdomen showing extensive conglomerate tumour masses with irregular margins and heterogeneous attenuation, encasing and displacing peritoneal structures.
Dual agent immunotherapy with ipilimumab and nivolumab was commenced but there was disease progression after four cycles and worsening obstructive symptoms. A second opinion was requested by the family and resulted in referral to our unit at The St George Hospital. A biopsy of the pelvic mass in July 2024 confirmed adenocarcinoma cells including signet cells within abundant extracellular mucin. In particular, it was noted that the tumour cells were predominantly necrotic and degenerate with few, if any, viable tumour cells and absent SATB2 expression, which had been strongly positive initially. This was in keeping with significant treatment response. Following extensive family and MDT discussions, surgical intervention was planned, with the intention of treating this as akin to pseudomyxoma peritonei (PMP) rather than colorectal malignancy with peritoneal metastasis.
In August 2024, the patient underwent CRS and HIPEC (Mitomycin-C), including total parietal peritonectomy, en-bloc resection of the pelvic mass, splenectomy, cholecystectomy, completion omentectomy and colectomy with end ileostomy (PCI 31, CC0). A total 10 kg of tumour was resected (Fig. 2), with heavy burden of disease involving the transverse and left colon, mesentery, right diaphragm, liver, and spleen.
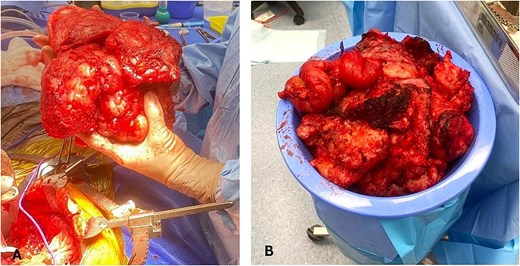
(A) Intraoperative image showing the en-bloc excision of a pelvic tumour mass with heterogeneous and lobulated morphology. (B) Extensive tumour specimen resected weighing ~10 kilograms.
He completed 4 days of early postoperative intraperitoneal chemotherapy (EPIC) with 5-FU as per The St George Hospital protocols without complication and was stepped down from the paediatric ICU on postoperative Day 8. Recovery was complicated by an intra-abdominal collection managed with antibiotics and radiological drainage, and gastroparesis requiring serial endoscopic dilations. With comprehensive multidisciplinary support, he was discharged home on postoperative Day 52.
Discussion
Large multicentre studies have demonstrated that patients with mucinous colonic adenocarcinoma experience poorer outcomes compared to those with non-mucinous tumours, showing lower median overall survival (13.2 vs. 19.2 months) and reduced 5-year disease-free survival (83.2%–87.4% vs. 83.2%–87.4%) following surgery and adjuvant therapy [5, 6].
The Peritoneal Surface Oncology Group International (PSOGI) endorses CRS-HIPEC as a safe and effective approach for treating peritoneal carcinomatosis of colorectal origin, provided the procedure is carried out in experienced, high-volume centres [7]. Although large-scale data on the long-term efficacy of CRS-HIPEC for MCA remains limited, Huang et al. found no significant difference in overall survival at 1, 3, and 5 years (88.6%, 51.8%, and 33.7%, respectively) or in median disease-free survival (13.1 months) between mucinous and non-mucinous groups following CRS-HIPEC [8]. These findings suggest that mucin is neither a negative prognostic factor nor a contraindication to surgery [8]. Similarly, Langner et al. reported that although mucinous adenocarcinomas were more frequently associated with mismatch repair deficiency, the presence and extent of extracellular mucin had no prognostic impact; instead, tumour grade and depth of invasion were more critical determinants of outcome [9].
In contrast, PMP is a rare peritoneal malignancy characterized by diffuse mucin accumulation, most often originating from appendiceal primaries [7, 8]. CRS-HIPEC has been shown to provide favourable long-term outcomes in PMP, even among patients with high peritoneal cancer index (PCI) scores, when complete cytoreduction is achieved [7, 8]. Large multicentre studies report a median overall survival of 196 months and a progression-free survival of 98%, with 10-year and 15-year survival rates of 63% and 59%, respectively [10]. These outcomes are consistent with international data and support the PSOGI recommendation that CRS-HIPEC, rather than repeated debulking, should be the treatment of choice in suitable PMP patients [7, 10–12].
This case also posted significant perioperative and management challenges, particularly in determining which institution would oversee the patient’s care across the perioperative period. Our hospital had experience with CRS-HIPEC and appropriate ICU facilities for adults but was unable to provide paediatric ICU support. Conversely, the referring paediatric hospital lacked experience in cytoreductive surgery and HIPEC.
To overcome this, a collaborative approach was adopted, effectively ‘bringing the hospital to the patient.’ Surgeons, anaesthetists, nursing teams and high-level management staff from our centre worked alongside staff from the referring hospital. This coordinated effort was enabled by comprehensive planning, inter-hospital communication, and joint preparation including educational sessions, operative rehearsals, and multidisciplinary discussions across surgical, anaesthetic, nursing, and allied health teams, ensuring alignment on CRS-HIPEC protocols and paediatric critical care needs. For the procedure, a combined adult-paediatric theatre team was formed including specialist peritonectomy anaesthetists working alongside specialist paediatric anaesthetists, the patient’s treating paediatric surgeon alongside the peritonectomy surgical team and a combined theatre/scrub team from both sites. The specialized peritonectomy nurse practitioners undertook education of the paediatric ICU nurses with regards to EPIC delivery and attended to facilitate treatment. Postoperative care was overseen by the paediatric surgical team, with the peritonectomy team attending at least daily in the immediate postoperative period and providing remote advice and in-person reviews as needed once beyond this stage.
Ultimately, pseudomyxoma peritonei and MCA are distinct entities with different biological behaviours. In this case, the patient developed new mucinous features following immuno-chemotherapy which likely reflect tumour evolution and mutation in setting of microsatellite instability and MMR deficiency. Given his age, disease progression, symptomatic disease, and the possibility of achieving meaningful disease control, we chose to proceed with surgery via CRS-HIPEC and treat the case as PMP. Ultimately, the decision was made following extensive discussion and counselling with the family with to alleviate symptoms and give the patient a chance at life.
Conclusion
Several key takeaways can be drawn from this case. First, although challenging, CRS-HIPEC can be a viable option for extensive peritoneal involvement in selected paediatric patients with appropriate pathology, even with a high PCI. Second, the success of this complex surgical intervention depended on meticulous planning and multidisciplinary collaboration across two hospitals, each with distinct strengths. Overcoming logistical challenges and institutional barriers, was crucial in delivering optimal care for the patient. This experience emphasizes the value of breaking down institutional barriers and engaging a diverse team of experts to provide innovative and patient-centred care, especially in rare and complex oncologic cases.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding
None declared.
Consent for publication
Written informed consent was obtained from the patient for publication of the details of their medical case and any accompanying images.



