-
PDF
- Split View
-
Views
-
Cite
Cite
Hidenobu Akamatsu, Daisuke Takeyoshi, Tasuku Kawarabayashi, Akito Inoue, Jeonga Lee, Jun Maruoka, Yuki Setogawa, Ryohei Ushioda, Ryo Okubo, Hiroyuki Miyamoto, Shougo Takahashi, Shingo Kunioka, Hiroyuki Kamiya, Successful limb salvage in acute type A aortic dissection with bilateral lower limb malperfusion by early cardiopulmonary bypass reperfusion and total arch replacement with frozen elephant trunk, Journal of Surgical Case Reports, Volume 2025, Issue 10, October 2025, rjaf837, https://doi.org/10.1093/jscr/rjaf837
Close - Share Icon Share
Abstract
We report a case of a 74-year-old man with acute type A aortic dissection (AADA) complicated by bilateral lower limb and renal malperfusion. Due to preoperative motor and sensory deficits, bilateral axillo-bifemoral grafts were used for arterial cannulation to initiate cardiopulmonary bypass and enable immediate limb reperfusion. Total arch replacement with a frozen elephant trunk was performed. To maintain lower limb perfusion perioperatively, a prophylactic ascending aorta-to-bilateral femoral artery bypass was constructed using the same grafts. Although the aorta-to-bilateral femoral artery bypass occluded within 1 week postoperatively due to true lumen expansion, limb perfusion was preserved, and no further revascularization was required. Continuous renal replacement therapy was initiated immediately postoperatively to prevent myonephropathic metabolic syndrome, leading to full renal recovery. The patient was discharged without neurological deficits or limb loss. This case underscores the effectiveness of early cardiopulmonary bypass reperfusion, temporary bypass, and renal support in AADA with limb malperfusion.
Introduction
Acute type A aortic dissection (AADA) is a life-threatening condition that requires emergency surgical intervention. Among its complications, malperfusion syndrome is a major determinant of prognosis, involving various organs such as the brain, coronary arteries, visceral organs, and extremities. Bilateral lower limb malperfusion, in particular, is associated with poor outcomes due to its frequent coexistence with other organ ischemia and the large volume of muscle at risk for necrosis [1, 2]. When lower limb ischemia persists for more than 4 to 6 h, the risk of irreversible neuromuscular damage and myonephropathic metabolic syndrome (MNMS)—including rhabdomyolysis and acute kidney injury—increases substantially, potentially leading to multi-organ failure [3]. Therefore, rapid and appropriate reperfusion strategies are crucial to improve outcomes. Here, we report a rare case of AADA complicated by bilateral lower limb malperfusion, in which limb salvage was successfully achieved through early cardiopulmonary bypass (CPB) reperfusion, total arch replacement with a frozen elephant trunk (TAR with FET), and early postoperative renal replacement therapy.
Case presentation
A 74-year-old man with a history of hypertension, chronic kidney disease, and a distal aortic arch aneurysm presented with sudden-onset bilateral leg weakness and sensory loss. On admission, femoral, popliteal, and pedal pulses were absent bilaterally. Contrast-enhanced computed tomography (CT) revealed AADA with a saccular aneurysm in the distal aortic arch and severe true lumen narrowing at the abdominal aorta, resulting in right renal and lower limb malperfusion (Fig. 1a–c).
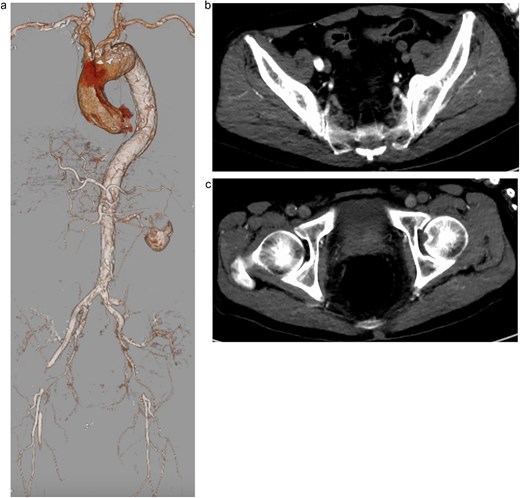
Preoperative contrast-enhanced computed tomography (CT) showing severe narrowing of the true lumen at the abdominal aorta, with dynamic obstruction of both renal arteries and static obstruction of the left external iliac and right common femoral arteries. (a) Three-dimensional reconstruction, (b) axial CT image at the level of iliac arteries, and (c) axial CT image at the level of femoral arteries.
Emergency surgery was performed, consisting of TAR using a 26-mm J Graft (Japan Lifeline Inc., Tokyo, Japan) and insertion of a 29 × 120 mm Frozenix® J-graft (Japan Lifeline Inc., Tokyo, Japan). Eight-mm PROPATEN vascular ring graft (W.L. Gore & Associates, Flagstaff, AZ, USA) was anastomosed to the left axillary artery and the other bifurcated PROPATEN graft was anastomosed to the bilateral femoral arteries via subcutaneous tunneling. CPB was initiated via all three sites, allowing for immediate reperfusion of the lower limbs. After CPB weaning, the proximal site of the bifurcated vascular graft, which had been anastomosed to the bilateral femoral arteries, was brought to the mediastinum via subcutaneous routing and anastomosed to a side branch of the J-Graft previously used for arterial cannulation, thereby completing a prophylactic ascending aorta-to-bilateral femoral artery (Ao-F) bypass.
The patient was extubated on postoperative day (POD) 3, and neurological examination revealed no abnormalities, with intact motor and sensory function in the lower limbs. To prevent MNMS, continuous renal replacement therapy (CRRT) was initiated immediately after surgery. Although MNMS was successfully avoided, renal dysfunction persisted, requiring temporary dialysis until POD 19. At the last follow-up, renal function had improved to preoperative levels, with a serum creatinine of 1.96 mg/dl and an estimated glomerular filtration rate of 26.9 ml/min/1.73 m2. Postoperative CT imaging revealed occlusion of the Ao-F bypass due to expansion of the true lumen; however, perfusion of the lower limbs and the right kidney, which had been malperfused preoperatively, was well preserved (Fig. 2).
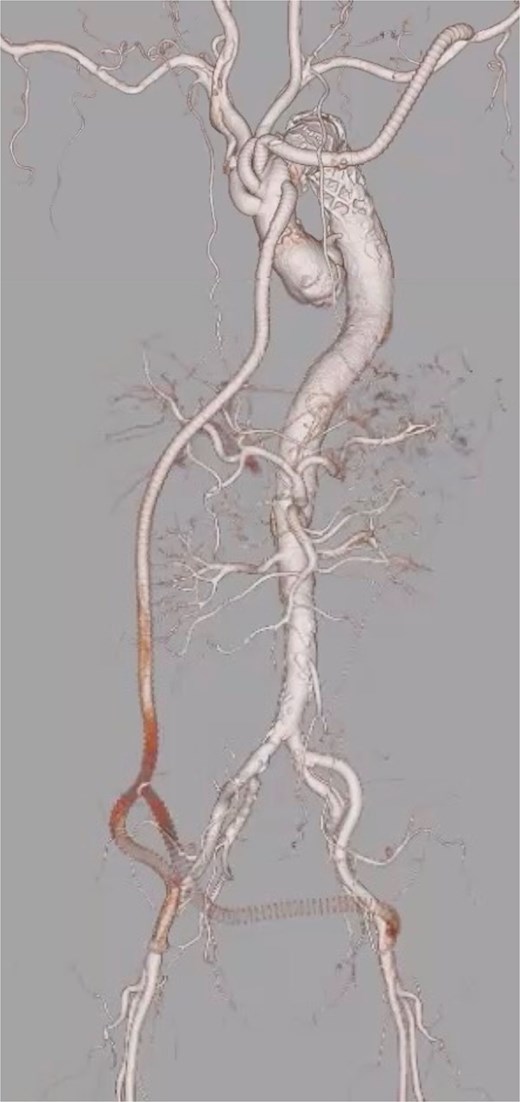
Postoperative contrast-enhanced 3D computed tomography demonstrating successful total arch replacement with frozen elephant trunk insertion and ascending aorta-to-bilateral femoral artery (Ao-F) bypass. Although the Ao-F bypass graft is occluded, lower limb perfusion is preserved via expansion of the true lumen following central repair.
Discussion
Lower limb malperfusion occurs in ~15%–17% of patients with AADA [4] and is associated with significantly increased hospital mortality [5, 6]. This is mainly due to concurrent malperfusion of other organs and systemic complications such as MNMS following limb reperfusion. Therefore, early and comprehensive intervention is essential to improve outcomes in these high-risk patients.
Understanding whether malperfusion is caused by dynamic or static obstruction is crucial for selecting the appropriate treatment strategy in acute aortic dissection [7]. Dynamic obstruction occurs when elevated false lumen pressure causes collapse of the true lumen, and it is often resolved by central aortic repair alone. In contrast, static obstruction results from anatomical compromise of branch vessels due to extension of the dissection flap, and typically necessitates additional revascularization procedures such as endovascular stenting or surgical bypass.
According to the Society for Vascular Surgery (SVS) classification, category I limbs require revascularization within 24 h, whereas categories IIa and IIb necessitate intervention within 6 h to prevent irreversible damage [8]. In the present case, although CPB could theoretically have been established via left axillary artery cannulation alone, preoperative CT demonstrated severe narrowing of the true lumen at the abdominal aorta, suggesting dynamic obstruction, while static obstruction at the iliac and femoral arteries could not be excluded. Therefore, more reliable lower limb reperfusion was deemed necessary. In addition, the patient presented with SVS category IIb ischemia, indicating an immediately threatened limb, and thus early restoration of perfusion was essential. For these reasons, bilateral femoral artery cannulation was planned preoperatively and promptly performed to ensure direct lower limb perfusion. Furthermore, based on the preoperative CT findings, we had already decided to construct a prophylactic ascending Ao-F bypass to secure lower limb perfusion during the perioperative period. Although the Ao-F bypass occluded within 1 week postoperatively due to true lumen expansion, it contributed to maintaining effective lower limb perfusion during the critical perioperative phase, and no further revascularization was necessary.
The risk of MNMS is particularly high in cases of prolonged limb ischemia. Reperfusion injury can lead to skeletal muscle necrosis, hyperkalemia, myoglobinuria, acute kidney injury, and eventually multi-organ failure. Recent studies have shown that early initiation of CRRT is effective in preventing MNMS in such patients [9, 10]. In this case, CRRT was introduced immediately after surgery, which successfully prevented the onset of MNMS and facilitated recovery of renal function to preoperative levels. The combination of timely CPB-assisted reperfusion and perioperative renal support enabled successful salvage of both lower limbs without the need for additional revascularization or amputation.
Conflict of interest statement
None declared.
Funding
None declared.
References
- aorta
- cardiopulmonary bypass
- metabolic syndrome x
- proximal aortic dissection
- reperfusion therapy
- physiologic reperfusion
- elephants
- limb
- femoral artery
- limb salvage
- perfusion
- preoperative care
- tissue transplants
- kidney
- leg
- bypass
- hypoperfusion
- revascularization
- trunk structure
- continuous renal replacement therapy



