-
PDF
- Split View
-
Views
-
Cite
Cite
Kira Carlotta Steinkraus, Michael Mühlberger, Stefan Andreas Schmidt, Marko Kornmann, Acinar cystic transformation of the pancreas—a rare case in a young patient, Journal of Surgical Case Reports, Volume 2023, Issue 2, February 2023, rjad077, https://doi.org/10.1093/jscr/rjad077
Close - Share Icon Share
Abstract
Acinar cystic transformation (ACT) is a very rare transformation of the pancreas and has been described in less than 100 cases since its first report in 2002. The aim of this case report is to get a better understanding of this pancreatic transformation, which to date appears to be non-malignant. However, radical surgery was performed in most cases due to misinterpreting the initial diagnosis. ACT may be misdiagnosed for intraductal papillary mucinous neoplasms and is currently not included as a potential differential diagnosis for cystic lesions of the pancreas. ACT belongs to the benign cystic alterations of the pancreas. Despite its rarity, it should be considered as a potential differential diagnosis with regard to cystic lesions in the pancreas, especially in order to avoid unnecessary surgery.
INTRODUCTION
Acinar cystic transformation (ACT), also referred to as acinar cell cystadenoma, is a very rare non-neoplastic transformation of the pancreas. It was first reported in 2002 as an incidental finding in an autopsy by Albores-Saaveda and described as an acinar cystadenoma [1]. The cystic lesions measured up to 19.7 cm and occurred as multilocular or unilocular cystic lesions [2]. Microscopically, the lesions were characterised by cysts of different sizes, lined by epithelium with regions of either acinar or ductal differentiation without nuclear atypia or mitoses. Rift et al. published in the first literature review that ACT occurred predominately in female patients with a mean age of 44.8 years diagnosed with abdominal pain and cystic lesions in the head of the pancreas [3].
Immunohistochemistry is needed to confirm the distinct regions of acinar and ductal differentiation and to rule out differential diagnoses such as malignant neoplasms, mucinous cystic neoplasms or intraductal papillary mucinous neoplasms (IPMN) [4, 5].
Most ACT cases have been thought to be IPMN prior to histologic confirmation. There is a 12% risk for invasive carcinoma within 60 months for patients with high-risk IPMN [6, 7], which explains why most patients receive surgery prior to accurate diagnosis.
However, a recent review of published cases regarding ACTs stated that no malignant transformation was observed [8].
CASE REPORT
A 37-year-old male patient referred himself with upper abdominal pain and general weakness in 2021. His previous medical history included an insulin-dependent diabetes mellitus, arterial hypertension, hepatic steatosis grade I, the condition after kidney transplant during childhood due to an autosomal recessive polycystic kidney disease and recently an acute on chronic prerenal kidney failure. In 2017 a magnetic resonance imaging (MRI) with cholangiopancreatography (MRCP) was performed due to cholestasis and elevated γ-glutamyl transferase, leading to the incidental diagnosis of a multiple branch IPMN.
At the presentation in 2021, ultrasonography diagnosed a gallbladder hydrops, but no cause could be delineated for this. Therefore, an MRI with MRCP was performed again leading to the diagnosis of a progressive side branch IPMN with possible focal involvement of the main duct and partial irregular cystic changes, as shown in Fig. 1. Additionally, an endosonography of the pancreas was carried out revealing the image morphology of a combined IPMN involving the main duct and showing progressive side branches with worrisome features shown in Fig. 2. The case was discussed in our multidisciplinary tumor board recommending the evaluation for a pancreatectomy due to high-risk IPMN.
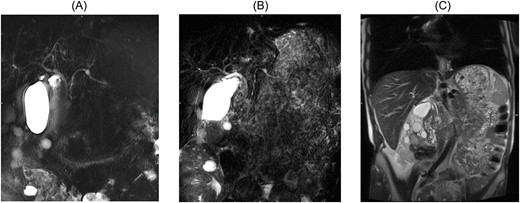
(A) MRCP 2017 displaying side branch IPMN with dilatation of the side branch, (B) MRCP 2021 displaying main duct involvement of the pancreas, (C) coronary MRT T2 scan with progressive side branch IPMN measuring 14 mm with possible focal discrete involvement of the main duct and partly irregular cystic changes.
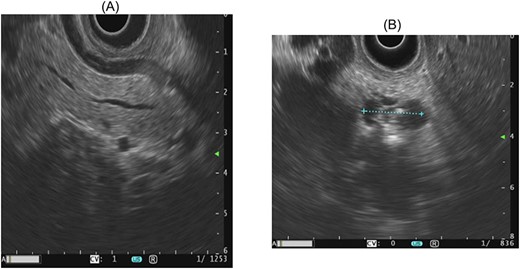
Endosonographic image morphology of a main duct IPMN, as well as progressive side branches with worrisome features, including dilatation of the main pancreatic duct and abrupt changes in the diameter.
For this reason, a robotic spleen-preserving, total pancreaticoduodenectomy with end-to-side hepaticojejunostomy and retrocolic duodenojejunostomy was performed. Post-operative complications did not occur and the patient was dismissed on post-operative day 10.
Histopathological examination of the pancreatic specimen showed a pronounced lymphoplasma cellular inflammation and was considered to be a chronic atrophic pancreatitis with no evidence of malignancy. Further histological cross-sections showed that the lesion was composed of multilocular cysts in the entire pancreas measuring up to 1.1 cm, shown in Fig. 3. Microscopically, the cysts were aligned by epithelium with regions of either acinar shown in Fig. 4 or ductal differentiation shown in Fig. 5, without nuclear atypia or mitoses. The surrounding parenchyma was atrophic and fibrotic. Immunohistochemical stains confirm the distinct regions of acinar differentiation (positive for BCL10, trypsin and chymotrypsin) shown in Fig. 4(B–D) and those of ductal differentiation (negative for BCL10, trypsin and chymotrypsin) shown in Fig. 5(B–D), leading to the final diagnosis of ACT.
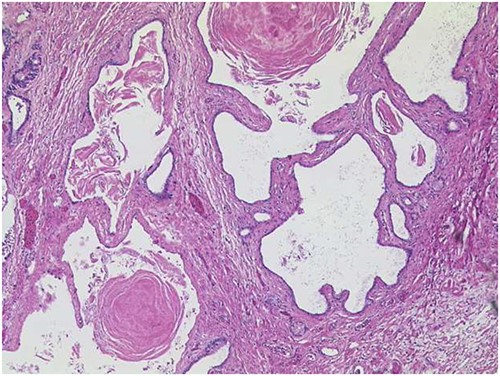
ACT of the pancreas with cysts of varying sizes, surrounded by fibrotic pancreatic parenchyma (HE ×50). On cross-section, the lesion is composed of multilocular cysts in the entire pancreas, measuring up to 1.1 cm.
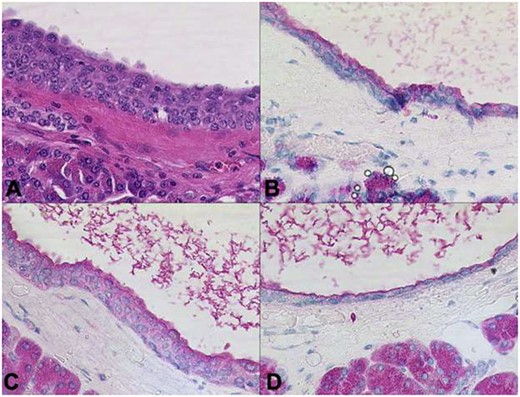
Cysts aligned by epithelium with acinar differentiation. The cyst lumina contain enzymatic secretions. Immunohistochemical stains confirm the acinar differentiation (A: HE, B: trypsin, C: chymotrypsin, D: BCL10 x200).
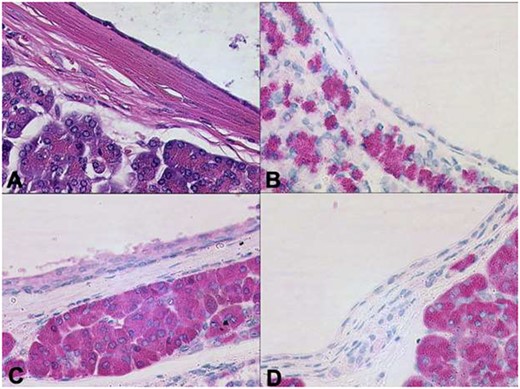
Distinct regions of ductal differentiation lack the immunohistochemical evidence of acinar cell markers (A: HE, B: trypsin, C: chymotrypsin, D: BCL10 x20).
DISCUSSION
Definite diagnosis of ACT is achieved by histopathological, histochemical and immunohistochemical results [9]. As no malignancy has been observed for any ACT patient in the current literature, it is possible to take a biopsy for histological interpretation followed by active surveillance, which might be a better approach rather than surgical intervention for the patient. The value of pre-operative biopsy depends among other things on the size of the biopsy specimen. Inappropriate biopsy specimen might not reveal the acinar epithelium and might lead to the diagnosis of a chronic pancreatitis.
However, to ensure ACT diagnosis prior to radical surgery, a new histology approach using a transgastric endoscope puncturing of a pancreas cyst with a 19-gauge needle for a confocal endomicroscopy probe has been used to identify acinar components. A biopsy forceps has been put through the needle lumen for biopsy under endoscopic ultrasound [10].
A link between polycystic kidney disease and occurring pancreatic cystic lesions has been described, although pancreatic cysts occur more frequently in patients with autosomal dominant polycystic kidney disease than in the autosomal recessive form [11].
In this case, a total pancreatectomy was performed due to the assumption of a high-risk IPMN. Current data are inconsistent regarding the need for pancreaticoduodenectomy or total pancreatectomy for main duct IPMN [12]. Based on the concomitant severe side diseases with the need of immunosuppressive medication and the already present insulin-dependent diabetes, a total pancreatectomy was performed. This strategy avoided a pancreatic anastomosis and its possible complications. For other patients, a partial pancreaticoduodenectomy would have been an alternative choice.
Pancreatic ACT leaves diagnosis at a challenge prior to surgery, as, to our knowledge, there is currently no method of modern imaging that can reliably distinguish ACT from other tumor entities. Therefore, a combination of different imaging modalities in combination with biopsy is currently recommended. Surgery for ACT in asymptomatic patients should be avoided, as it is associated with possible morbidity and mortality and loss in quality of life. In addition, surgery for symptomatic ACT could be performed as a parenchyma-sparing form, e.g. as enucleation.
More importantly, the list of cystic pancreatic lesions has to be expanded for ACT. Cystic pancreatic lesions include pseudocysts, common cystic neoplasms like IPMNs, serous cystic neoplasms (SCN) and mucinous cystic neoplasms (MCN), uncommon cystic neoplasms like solid pseudopapillary epithelial neoplasms (SPEN), dermoid cysts and tumors (adenocarcinoma/neuroendocrine carcinoma) with cystic degeneration [13, 14]. ACT should be presently included as a member of the latter group. Whereas SPEN are seen in young, MCN in middle aged and SCN in older women, the gender- and age-specific frequency of ACT has to be determined as well [14].
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
STATEMENT OF ETHICS
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
References
Nagtegaal ID, Odze RD, Klimstra D, Paradis V, Rugge M, Schirmacher P, et al.



