-
PDF
- Split View
-
Views
-
Cite
Cite
Yasuhiro Ishiyama, Misato Ito, Sohei Akuta, Masatoshi Yoshizawa, Misuzu Yamato, Hiroto Tanaka, Takatsugu Fujii, Naoto Okazaki, Chikashi Hiranuma, Katsuya Deguchi, Yasumitsu Hirano, Small bowel fistula with colorectal cancer and mesenteric lymph node metastasis: a report of two cases, Journal of Surgical Case Reports, Volume 2023, Issue 12, December 2023, rjad675, https://doi.org/10.1093/jscr/rjad675
Close - Share Icon Share
Abstract
A 65-year-old man presented to our hospital with complaints of diarrhea. Computed tomography showed a fistula with the small intestine, and a single incision laparoscopic low anterior resection for rectum with D3 dissection and partial resection of the small intestine were performed. Lymph node dissection, including a part of the inflow vessel area, was also performed because lymph node swelling was observed in the mesentery of the small intestine around the fistula. Histopathological analysis revealed that the lymph nodes in the small intestine were positive for metastasis. The patient was a 61-year-old woman who presented to our hospital with a chief complaint of diarrhea. A partial resection of the small intestine, including resection of the left hemicolectomy and lymph node dissection around the fistula, was performed at laparotomy. Histopathological examination revealed numerous lymph node metastases in the small intestinal mesentery.
Introduction
Although it is recommended that colorectal cancer (CRC) be detected early and treated as soon as possible, it is not uncommon to detect obstructive CRC due to symptoms or CRC that has already invaded other organs [1].
Unfortunately, there have been only a few case reports of small bowel mesenteric lymph node metastasis in patients with CRC with small bowel invasion [2, 3], and the low frequency of small bowel mesenteric lymph node metastases makes it debatable whether systematic resection of small bowel lymph nodes should be performed in all patients with CRC with small bowel invasion.
In this report, we describe two cases of direct invasion of the small intestine by CRC and fistula formation, in which lymph node dissection of the mesentery of the small intestine was performed, and histopathological examination revealed metastasis in the lymph nodes of the small intestine.
Case report
Case 1
The patient was a 65-year-old man presenting to our hospital with complications of diarrhea for a year and weight loss. Colonoscopy showed rectal cancer on the anal verge (13 cm) with circumferential stenosis. Laboratory findings showed that the serum carcinoembryonic antigen (CEA) level was 4.7 μg/ml and that the serum carbohydrate antigen 19-9 (CA19-9) was 72.2 U/ml. A computed tomography (CT) scan of the abdomen showed thickening of the rectal wall with inflammation. Fistula formation between the ileum and the rectum was observed. Enlarged lymph nodes were also noted around the rectum and no enlarged mesenteric lymph nodes (Fig. 1). The patient was diagnosed with rectal cancer with ileum invasion and then scheduled to undergo surgery. We performed single-incision laparoscopic low anterior resection of the rectum with D3 dissection and partial resection of the ileum. Lymph node dissection, including the area of the inflow vessel, was also performed due to observed lymph node swelling in the mesentery of the ileum around the fistula formation intraoperatively. The operative time was 326 min, and the blood loss volume was 15 ml. Histopathological analysis revealed that the lymph nodes in the small intestine were positive for metastasis, and the diagnosis was pT4b (small intestine) N1 (2/16 (#251, 1/12, #252 1/4, #253 0/0)) M1 (small intestine mesenteric lymph node (1/1)), nonsolid type (por2) adenocarcinoma, ly3, v2 f Stage IV [TNM (tumor, nodes, metastasis) classification] (Fig. 2a and b). After discharge from the hospital, capecitabine plus oxaliplatin was administered for 6 months as adjuvant chemotherapy. The patient has been recurrence-free for 2 years after surgery.
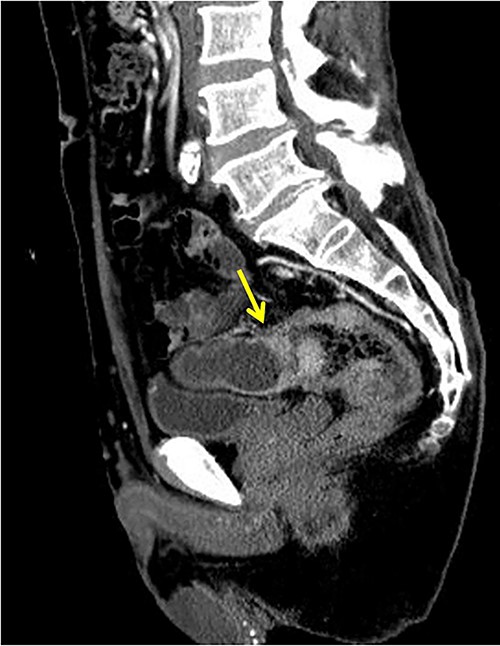
Fistula formation between the small intestine and the rectum was observed. Enlarged lymph nodes were also noted around the rectum.
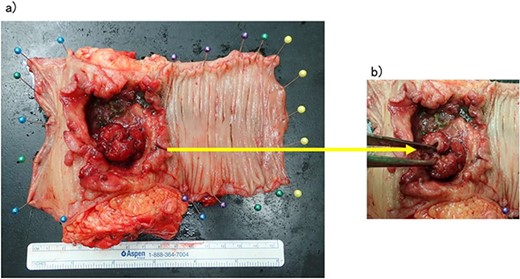
(a, b) Rectal cancer with small intestine invasion and fistula formation.
Case 2
The patient was a 61-year-old woman presenting to our institution with the chief complaint of abdominal distension and diarrhea for a month. Colonoscopy showed descending colon cancer with all circumference-related stenosis. Her laboratory findings revealed a serum CEA level of 5.5 μg/ml and serum CA19-9 level of 778.4 U/ml. The CT scan results showed wall thickening in the descending colon. The descending colon on the proximal side of the tumor was dilated. The ileum is in contact with the tumor. Positron emission tomography (PET-CT) showed fluorodeoxyglucose (FDG) accumulation in the descending colon, mediastinal lymph nodes, enlarged mesenteric lymph nodes, and pararenal aortic lymph nodes (Fig. 3). The patient was preoperatively diagnosed as Stage IV, but she had obstructive symptoms, so surgery was planned. Laparotomy with left hemicolectomy and partial small bowel resection were performed. The ileum, including lymph node dissection around the fistula formation, was also resected.
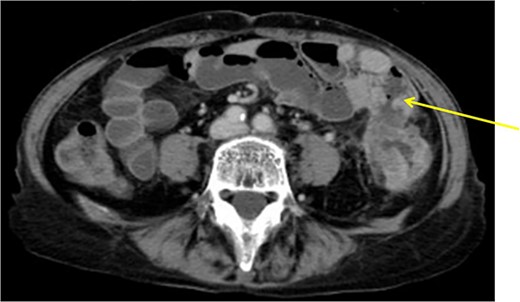
Descending colon with circumference tumor and small intestine invasion.
The operative time was 234 min, and the blood loss volume was 155 ml. Histopathological analysis revealed numerous lymph node metastases within the mesentery of the small intestine, and the patient was diagnosed with pT4b (small intestinal mucosa) N3 (28/41) M1 (small mesenteric lymph node 6/8) f Stage IV (TNM classification), nonsolid type (por2) adenocarcinoma, ly3, v2 (Fig. 4a and b). After receiving chemotherapy, she died 18 months after surgery.
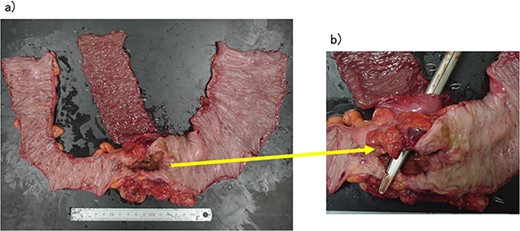
(a, b) Descending colon cancer with small intestine invasion and fistula formation.
Discussion
The frequency of fistula in colon cancer patients is very low. Welch et al. [4] reported that in only 13 among 2004 cases. The underlying mechanism of fistula formation is that adhesion to adjacent organs must occur prior to tumor invasion and that cancer must invade the adjacent organs within the adherent area. First, inflammation spreads around the colon due to destruction of the colonic wall structure by the cancer [5], and then a fistula can form due to the invasion of adjacent organs by the colon tumor and associated tissue necrosis [6].
On the other hand, the guidelines of the Japanese Society for Cancer of the Colon and Rectum recommend complete mesocolic excision with central vessel ligation for colon cancer [7]. However, the necessity of lymph node dissection for invasion of other organs, including invasion of the small intestine, is not mentioned, and considering the tumor status.
We examined 52 cases of CRC with suspected small intestinal invasion during the period from 2007 to 2019 at Saitama Medical University International Medical Center. In terms of invasion into the small intestine, 16 cases were found to have progressed deeper than the mucosal lamina propria, with 2 cases showing small intestinal mesenteric lymph node metastasis (Table 1).
| n = 52 . | . |
|---|---|
| Age | 70 (31-88) |
| Sex | |
| Male | 28 |
| Female | 24 |
| Tumor location | |
| Cecum | 6 |
| Ascending | 6 |
| Transverse | 9 |
| Descending | 3 |
| Sigmoid | 19 |
| Rectal | 9 |
| Surgical approach | |
| Laparoscopic | 18 |
| Conversion to open surgery | 4 |
| Open | 34 |
| Tumor differentiation | |
| Tub1 | 5 |
| Tub2 | 31 |
| Muc | 4 |
| Por | 12 |
| Sig | 0 |
| Lymphatic invasion | |
| + | 18 |
| - | 34 |
| Venous invasion | |
| + | 36 |
| - | 16 |
| Invasion depth of small intestine | |
| Mucosa | 11 |
| Submucosa | 0 |
| Mucosa propria | 5 |
| Serous membrane | 4 |
| No invasion | 32 |
| n = 52 . | . |
|---|---|
| Age | 70 (31-88) |
| Sex | |
| Male | 28 |
| Female | 24 |
| Tumor location | |
| Cecum | 6 |
| Ascending | 6 |
| Transverse | 9 |
| Descending | 3 |
| Sigmoid | 19 |
| Rectal | 9 |
| Surgical approach | |
| Laparoscopic | 18 |
| Conversion to open surgery | 4 |
| Open | 34 |
| Tumor differentiation | |
| Tub1 | 5 |
| Tub2 | 31 |
| Muc | 4 |
| Por | 12 |
| Sig | 0 |
| Lymphatic invasion | |
| + | 18 |
| - | 34 |
| Venous invasion | |
| + | 36 |
| - | 16 |
| Invasion depth of small intestine | |
| Mucosa | 11 |
| Submucosa | 0 |
| Mucosa propria | 5 |
| Serous membrane | 4 |
| No invasion | 32 |
| n = 52 . | . |
|---|---|
| Age | 70 (31-88) |
| Sex | |
| Male | 28 |
| Female | 24 |
| Tumor location | |
| Cecum | 6 |
| Ascending | 6 |
| Transverse | 9 |
| Descending | 3 |
| Sigmoid | 19 |
| Rectal | 9 |
| Surgical approach | |
| Laparoscopic | 18 |
| Conversion to open surgery | 4 |
| Open | 34 |
| Tumor differentiation | |
| Tub1 | 5 |
| Tub2 | 31 |
| Muc | 4 |
| Por | 12 |
| Sig | 0 |
| Lymphatic invasion | |
| + | 18 |
| - | 34 |
| Venous invasion | |
| + | 36 |
| - | 16 |
| Invasion depth of small intestine | |
| Mucosa | 11 |
| Submucosa | 0 |
| Mucosa propria | 5 |
| Serous membrane | 4 |
| No invasion | 32 |
| n = 52 . | . |
|---|---|
| Age | 70 (31-88) |
| Sex | |
| Male | 28 |
| Female | 24 |
| Tumor location | |
| Cecum | 6 |
| Ascending | 6 |
| Transverse | 9 |
| Descending | 3 |
| Sigmoid | 19 |
| Rectal | 9 |
| Surgical approach | |
| Laparoscopic | 18 |
| Conversion to open surgery | 4 |
| Open | 34 |
| Tumor differentiation | |
| Tub1 | 5 |
| Tub2 | 31 |
| Muc | 4 |
| Por | 12 |
| Sig | 0 |
| Lymphatic invasion | |
| + | 18 |
| - | 34 |
| Venous invasion | |
| + | 36 |
| - | 16 |
| Invasion depth of small intestine | |
| Mucosa | 11 |
| Submucosa | 0 |
| Mucosa propria | 5 |
| Serous membrane | 4 |
| No invasion | 32 |
Histologically, 20 of the 52 cases showed invasion of the small intestine. In nine cases, tumor invasion was confined to the serosa in four cases and musclaris propria in five cases, and no lymph node metastasis to the small intestinal mesentery was observed in these cases. On the other hands, in two cases in which metastasis to lymph nodes in the mesentery of the small intestine was observed, both cases showed invasion deeper than the mucosal lamina propria and the formation of fistulas. These cases also showed lymph node metastasis to the mesentery at the primary site. In addition, combining the past literature and our own experience, six cases of small intestinal invasion of CRC with small mesenteric lymph node metastasis have been observed. Four of these cases had fistula formation in the small intestine (Table 2).
Cases of small intestinal invasion of CRC with small mesenteric lymph node metastasis.
| Case . | Authors . | Year . | Sex . | Age . | Primary . | Complain . | Differentiation . | Fistula . | Invaded organ . | Invasion depth of small intestine . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A Takiyama | 2016 | Woman | 80 | Sigmoid | Positive fecal occult blood test | − | Terminal ileum | Dead (31) | ||
| 2 | A Takiyama | 2016 | Man | 79 | Sigmoid | Constipation and weight loss | + | Jejunum | Dead (48) | ||
| 3 | A Takiyama | 2016 | Man | 76 | Ascending | Weight loss and appetite loss | − | Small bowel | Dead (67) | ||
| 4 | S Eto | 2021 | Man | 67 | Sigmoid | Weight loss and nausea | Tub2 | + | Ieum | Submucosa | Alive ((21) |
| 5 | Our case | 2022 | Man | 65 | Rectal | Weight loss and diarrhea | Por2 | + | Small bowel | Mucosa propria | Alive (24) |
| 6 | Our case | 2022 | Woman | 61 | Sigmoid | Diarrhea | Por2 | + | Small bowel | Mucosa | Dead (18) |
| Case . | Authors . | Year . | Sex . | Age . | Primary . | Complain . | Differentiation . | Fistula . | Invaded organ . | Invasion depth of small intestine . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A Takiyama | 2016 | Woman | 80 | Sigmoid | Positive fecal occult blood test | − | Terminal ileum | Dead (31) | ||
| 2 | A Takiyama | 2016 | Man | 79 | Sigmoid | Constipation and weight loss | + | Jejunum | Dead (48) | ||
| 3 | A Takiyama | 2016 | Man | 76 | Ascending | Weight loss and appetite loss | − | Small bowel | Dead (67) | ||
| 4 | S Eto | 2021 | Man | 67 | Sigmoid | Weight loss and nausea | Tub2 | + | Ieum | Submucosa | Alive ((21) |
| 5 | Our case | 2022 | Man | 65 | Rectal | Weight loss and diarrhea | Por2 | + | Small bowel | Mucosa propria | Alive (24) |
| 6 | Our case | 2022 | Woman | 61 | Sigmoid | Diarrhea | Por2 | + | Small bowel | Mucosa | Dead (18) |
Cases of small intestinal invasion of CRC with small mesenteric lymph node metastasis.
| Case . | Authors . | Year . | Sex . | Age . | Primary . | Complain . | Differentiation . | Fistula . | Invaded organ . | Invasion depth of small intestine . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A Takiyama | 2016 | Woman | 80 | Sigmoid | Positive fecal occult blood test | − | Terminal ileum | Dead (31) | ||
| 2 | A Takiyama | 2016 | Man | 79 | Sigmoid | Constipation and weight loss | + | Jejunum | Dead (48) | ||
| 3 | A Takiyama | 2016 | Man | 76 | Ascending | Weight loss and appetite loss | − | Small bowel | Dead (67) | ||
| 4 | S Eto | 2021 | Man | 67 | Sigmoid | Weight loss and nausea | Tub2 | + | Ieum | Submucosa | Alive ((21) |
| 5 | Our case | 2022 | Man | 65 | Rectal | Weight loss and diarrhea | Por2 | + | Small bowel | Mucosa propria | Alive (24) |
| 6 | Our case | 2022 | Woman | 61 | Sigmoid | Diarrhea | Por2 | + | Small bowel | Mucosa | Dead (18) |
| Case . | Authors . | Year . | Sex . | Age . | Primary . | Complain . | Differentiation . | Fistula . | Invaded organ . | Invasion depth of small intestine . | Outcome . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | A Takiyama | 2016 | Woman | 80 | Sigmoid | Positive fecal occult blood test | − | Terminal ileum | Dead (31) | ||
| 2 | A Takiyama | 2016 | Man | 79 | Sigmoid | Constipation and weight loss | + | Jejunum | Dead (48) | ||
| 3 | A Takiyama | 2016 | Man | 76 | Ascending | Weight loss and appetite loss | − | Small bowel | Dead (67) | ||
| 4 | S Eto | 2021 | Man | 67 | Sigmoid | Weight loss and nausea | Tub2 | + | Ieum | Submucosa | Alive ((21) |
| 5 | Our case | 2022 | Man | 65 | Rectal | Weight loss and diarrhea | Por2 | + | Small bowel | Mucosa propria | Alive (24) |
| 6 | Our case | 2022 | Woman | 61 | Sigmoid | Diarrhea | Por2 | + | Small bowel | Mucosa | Dead (18) |
In conclusion, if lymph node metastases are suspected due to enlarged lymph nodes observed intraoperatively or on preoperative CT scans, lymph node dissection of the mesentery might be considered.
Acknowledgements
All authors were edited for proper English language, grammar, punctuation, spelling, and overall style by one or more of the highly qualified native English speaking editors at AJE.
Conflict of interest statement
None declared.
Funding
None declared.
Author contributions
M.I., Y.I., K.D., and Y.H. designed the study and performed the experiments; Y.I., Y.H., T.F., and N.O. wrote the manuscript. Y.H. drafted the original manuscript. Y.H. supervised the conduct of this study. All authors approved the final version of the manuscript to be published.
Data availability
The data that support the findings of this study are available on request from the corresponding author, [Y.I.]. The data are not publicly available due to [restrictions, e.g. their containing information that could compromise the privacy of research participants].
Ethics approval and consent to participate
The study protocol was approved by the Ethics Committee of Saitama Medical University International Medical Center and conformed to the provisions of the Declaration of Helsinki. The study protocol was approved by the Institutional Review Board for the Use of Human Subjects at our institution, and written informed consent was obtained from all patients prior to study inclusion.
Consent for publication
All study participants provided informed consent by the appropriate ethics review board.



