-
PDF
- Split View
-
Views
-
Cite
Cite
Yasmine Laraqui, Youssef Mahdi, Hajar El Agouri, Mouna Khmou, Ismail Boujida, Khouloud Raissouni, Soumaya Echcharif, Basma El Khannoussi, Breast metastases from primary tumor of the urinary tract: case series, Journal of Surgical Case Reports, Volume 2023, Issue 1, January 2023, rjad017, https://doi.org/10.1093/jscr/rjad017
Close - Share Icon Share
Abstract
Breast metastasis from urological tract is exceptional, with a few sporadic cases reported in the literature. They can be confused with a primary breast cancer, especially in patients without clinical history, leading to an inappropriate and invasive treatment. Therefore, we have summarized some characteristics of metastatic breast tumors through this retrospective study.
INTRODUCTION
Breast metastasis represents less than 2% of malignant breast tumors; those of urological origin are exceptional, with a few sporadic cases reported in the literature. Due to their rarity and the nonspecific clinical and radiological features, they can be confused with a primary breast cancer. To avoid unnecessary and invasive treatments, an accurate diagnosis is essential. Therefore, we have summarized some characteristics of metastatic breast tumors by reviewing our cases.
MATERIAL AND METHODS
This is a retrospective study, extending between January 2017 and January 2022, carried out within the pathological anatomy laboratory of the National Institute of Oncology in Rabat, specializing in gyneco-mammary and digestive pathology. All patients, male and female, with a histological diagnosis of breast metastasis, were included.
RESULTS
Three out of 2390 cases (0.12%) have been identified in our laboratory over a period of 5 years: two men and a woman. All had a history of primary cancer originating from the urinary tract (urothelial bladder carcinoma, renal clear cell carcinoma and primitive renal leiomyosarcoma). The interval between the diagnosis of the primary tumor and the metastasis varied between 1 and 18 years. Patient history, histopathology and the immunohistochemical study confirmed the extramammary origin of the lesions.
CASES PRESENTATION (TABLE 1)
First case
We report the case of a 64-year-old man, chronic smoker, who presented with an isolated right breast nodule. The patient had 9 months previously undergone a radical cystoprostatectomy for a high-grade infiltrating urothelial carcinoma, infiltrating the bladder muscle, with the presence of a micropapillary component, and vascular invasion.
| Case N°1 | Case N°2 | Case N°3 | |
| Sex | M | F | M |
| Age | 64 | 69 | 58 |
| Primitive tumor | |||
| Site | Bladder | Kidney | Kidney |
| Histological type | High-grade infiltrating urothelial carcinoma | Clear cell carcinoma | Leiomyosarcoma |
| Interval between initial diagnosis and metastases | 9 months | 18 years | 2 years |
| Metastasis | |||
| Clinical signs |
| ||
| Radiological aspect | Subcutaneous tissue formation, echogenic, with irregular contours and without satellite adenopathy | ||
| Histological findings | – Carcinomatous proliferation – Spans, cords and some glandular structures – Atypical cells and richly mitotic | – Carcinomatous proliferation – Alveolar architecture – Cells with clear cytoplasm surrounded by distinct cell membranes | – Spindle cell proliferation – Long crisscrossed bundles – Moderate nuclear pleomorphism – Numerous figures of atypical mitoses |
| IHC study |
|
|
|
| Therapeutics | Lumpectomy of the right breast | Lumpectomy | Symptomatic treatment with radiological control |
| Evolution | Appearance of other secondary localizations after 6 months | Good outcome | Appearance of other secondary localizations |
| Case N°1 | Case N°2 | Case N°3 | |
| Sex | M | F | M |
| Age | 64 | 69 | 58 |
| Primitive tumor | |||
| Site | Bladder | Kidney | Kidney |
| Histological type | High-grade infiltrating urothelial carcinoma | Clear cell carcinoma | Leiomyosarcoma |
| Interval between initial diagnosis and metastases | 9 months | 18 years | 2 years |
| Metastasis | |||
| Clinical signs |
| ||
| Radiological aspect | Subcutaneous tissue formation, echogenic, with irregular contours and without satellite adenopathy | ||
| Histological findings | – Carcinomatous proliferation – Spans, cords and some glandular structures – Atypical cells and richly mitotic | – Carcinomatous proliferation – Alveolar architecture – Cells with clear cytoplasm surrounded by distinct cell membranes | – Spindle cell proliferation – Long crisscrossed bundles – Moderate nuclear pleomorphism – Numerous figures of atypical mitoses |
| IHC study |
|
|
|
| Therapeutics | Lumpectomy of the right breast | Lumpectomy | Symptomatic treatment with radiological control |
| Evolution | Appearance of other secondary localizations after 6 months | Good outcome | Appearance of other secondary localizations |
| Case N°1 | Case N°2 | Case N°3 | |
| Sex | M | F | M |
| Age | 64 | 69 | 58 |
| Primitive tumor | |||
| Site | Bladder | Kidney | Kidney |
| Histological type | High-grade infiltrating urothelial carcinoma | Clear cell carcinoma | Leiomyosarcoma |
| Interval between initial diagnosis and metastases | 9 months | 18 years | 2 years |
| Metastasis | |||
| Clinical signs |
| ||
| Radiological aspect | Subcutaneous tissue formation, echogenic, with irregular contours and without satellite adenopathy | ||
| Histological findings | – Carcinomatous proliferation – Spans, cords and some glandular structures – Atypical cells and richly mitotic | – Carcinomatous proliferation – Alveolar architecture – Cells with clear cytoplasm surrounded by distinct cell membranes | – Spindle cell proliferation – Long crisscrossed bundles – Moderate nuclear pleomorphism – Numerous figures of atypical mitoses |
| IHC study |
|
|
|
| Therapeutics | Lumpectomy of the right breast | Lumpectomy | Symptomatic treatment with radiological control |
| Evolution | Appearance of other secondary localizations after 6 months | Good outcome | Appearance of other secondary localizations |
| Case N°1 | Case N°2 | Case N°3 | |
| Sex | M | F | M |
| Age | 64 | 69 | 58 |
| Primitive tumor | |||
| Site | Bladder | Kidney | Kidney |
| Histological type | High-grade infiltrating urothelial carcinoma | Clear cell carcinoma | Leiomyosarcoma |
| Interval between initial diagnosis and metastases | 9 months | 18 years | 2 years |
| Metastasis | |||
| Clinical signs |
| ||
| Radiological aspect | Subcutaneous tissue formation, echogenic, with irregular contours and without satellite adenopathy | ||
| Histological findings | – Carcinomatous proliferation – Spans, cords and some glandular structures – Atypical cells and richly mitotic | – Carcinomatous proliferation – Alveolar architecture – Cells with clear cytoplasm surrounded by distinct cell membranes | – Spindle cell proliferation – Long crisscrossed bundles – Moderate nuclear pleomorphism – Numerous figures of atypical mitoses |
| IHC study |
|
|
|
| Therapeutics | Lumpectomy of the right breast | Lumpectomy | Symptomatic treatment with radiological control |
| Evolution | Appearance of other secondary localizations after 6 months | Good outcome | Appearance of other secondary localizations |
Clinically, it was a mammary nodule of hard consistency on palpation, mobile toward the deep plane, with no skin lesion, or nipple retraction or discharge. The lymph nodes were free. The patient underwent a breast ultrasound, which showed tissue formation subcutaneously, echogenic, with irregular contours, and discreetly vascularized by color Doppler, with the absence of satellite adenopathy. The contralateral breast showed no significant radiological abnormalities. The radiological assessment was completed by a thoraco-abdomino-pelvic CT scan, objectifying a poorly limited right breast mass, infiltrating the subcutaneous tissue, measuring 62 mm on the long axis, with no other secondary locations. Given that, final diagnosis is based on the clinico-radiological and anatomopathological confrontation.
The patient underwent a wide lumpectomy of the right breast, with respect to the surgical margins. The macroscopic examination showed a piece of lumpectomy, weighing 120 g, measuring 10 × 9 × 1.5 cm and seat of an ulceration of 7.5 × 7 cm. On section, this ulceration corresponded to a whitish mass, badly limited, indurated and reaching to the cutaneous plane. The histological study showed a mammary parenchyma infiltrated by a carcinomatous proliferation made up of spans, cords and some glandular structures. The cells are clearly atypical and richly mitotic. This tumor massively ulcerates the surface epidermis. No intra-ductal component or lymphovascular invasion was seen. The tumor was classified as pT4b. An immunohistochemical complement was necessary in order to determine the primary or secondary origin. It showed positive staining of tumor cells with anti-cytokeratin7, anti-cytokeratin 20 and anti-GATA3 antibodies. Hormone receptors (ER and PR), HER2 and anti-mammaglobin were negative. Thus, the diagnosis retained was that of localization in the breast of a poorly differentiated carcinomatous process compatible with the vesical origin already known in the patient (Fig. 1). The evolution was marked by the appearance of other secondary localizations after 6 months.
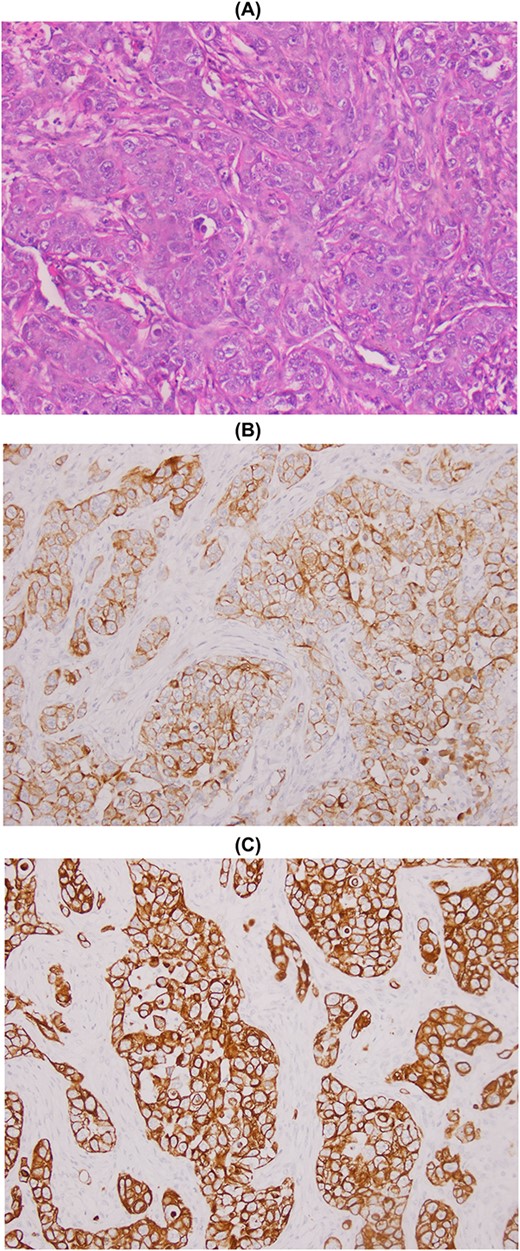
Metastasis of high-grade infiltrating urothelial carcinoma: poorly differentiated carcinomatous process (A), CK7+ (B) and CK20+ (C).
Second case
The patient was 69 years old and had been operated 18 years ago for a tumor of the right kidney, for which the diagnosis of clear cell carcinoma of the kidney was confirmed in the anatomopathological study of the surgical specimen. She was referred to our establishment for the management of a breast mass in the left upper outer quadrant, approximately 2.5 cm long axis, poorly limited and irregular.
The patient underwent a biopsy of the mass. Histological examination showed a carcinomatous proliferation with alveolar architecture composed of cells with clear cytoplasm surrounded by distinct cell membranes. The tumor nests are separated by a delicate vascular network (Fig. 2). Immunohistochemical staining shows PAX 8 antibody and CD10 antibody-positive staining for tumor cells, but negative for GATA3 and CK7 (Fig. 3). Thus, the diagnosis of breast localization of clear cell carcinoma of the kidney was made.
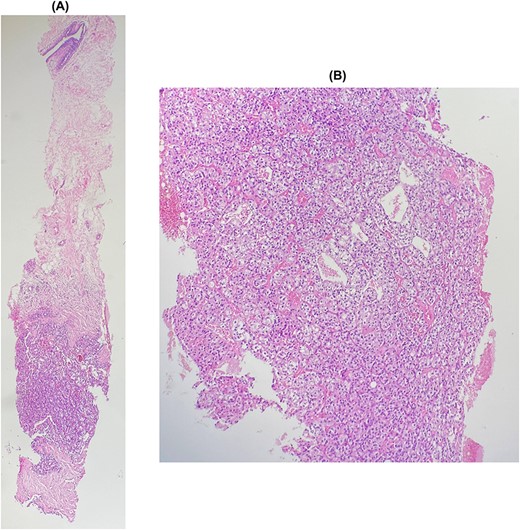
(A) and (B): Breast metastasis of clear cell carcinoma. Breast tissue infiltrated by a carcinomatous proliferation composed of nests, separated by a vascular network. Tumor cells show clear cytoplasm surrounded by distinct cell membranes.
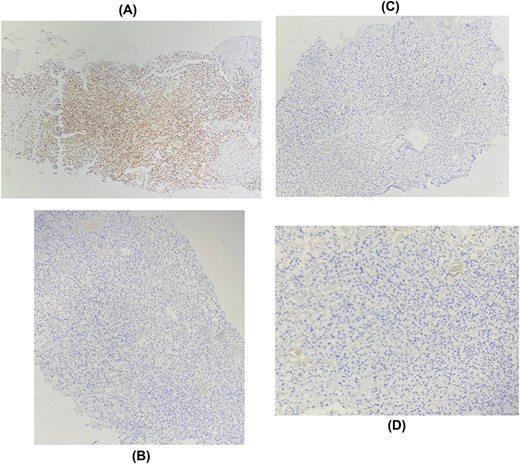
Immunohistochemical staining showing positive expression of PAX8 in tumor cells: (A) negative expression of GATA3 (B), RE (C), RP (D).
Third case
This is a 58-year-old patient, followed for two years for leiomyosarcoma of the right renal vein, with pulmonary and cervical spinal metastases, treated by surgery then adjuvant chemotherapy. The evolution was marked by the appearance of a right painful breast mass. Given the clinical context, the diagnosis of secondary localization of leiomyosarcoma was suggested on ultrasound and CT, and then confirmed on biopsy. The histological study shows a spindle cell proliferation made up of long crisscrossed bundles and showing a moderate nuclear pleomorphism with numerous figures of atypical mitoses (Fig. 4). The immunohistochemical study confirms the muscular origin by showing the positivity of the myogenic markers (SMA, Desmin, H-caldesmon) (Fig. 5).
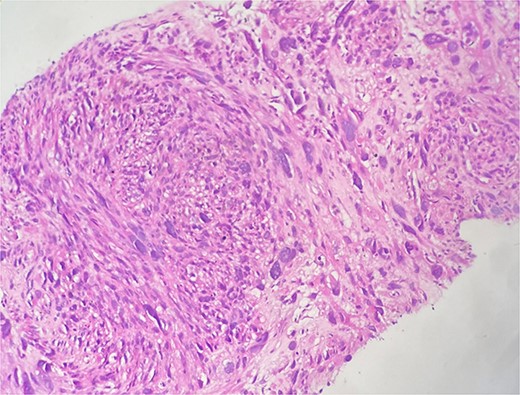
Microscopic examination shows fibro-fatty tissue infiltrated by a sarcomatous proliferation made up of long bundles of spindle-shaped cells of high cellular density. These cells have elongated, hyperchromatic nuclei with nuclear pleomorphism.
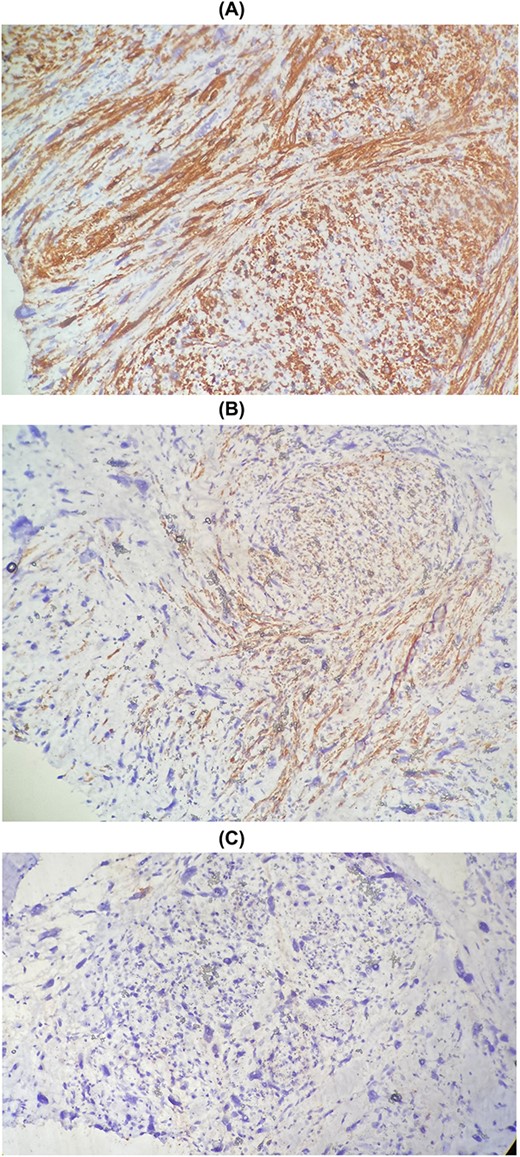
Immunohistochemical staining showing positive expression of H-caldesmon (A) and Desmin (B) negative expression of CKAE1/AE3 (C).
Only symptomatic treatment with radiological control was decided in this patient.
DISCUSSION
Breast metastases are rare; however, they must always be considered in the differential diagnosis of a breast lesion because of their poor prognosis and the need for different therapies from those used to treat primary breast cancer [1].
In addition to contralateral breast cancer metastases, the most common primary tumors with breast metastases are hematological malignancies, including leukemia and lymphoma, melanoma, lung cancer, ovarian cancer, gastric cancer and neuroendocrine cancer and in men, prostate cancer [2, 3]. The proportions of each vary in the literature, but those of urological origin remain exceptional. Only rare cases have been described, and mainly involved women [4]. They generally occur between the ages of 50 and 60 in patients with a known history of cancer, which is consistent with our series. Metastases are usually detected 1 to 10 years after the initial cancer diagnosis. In our cases, the median interval between initial diagnosis and metastases was 7 years. In 11% of cases, the breast lesion is revealing [5].
Histological diagnosis by fine-needle biopsy remains essential for the diagnosis of newly detected breast cancer, even in the presence of a history of extramammary cancer. Nevertheless, some clinical and radiological characteristics can help in the differential diagnosis.
Clinically, it is a mobile, palpable, painless, rapidly growing mass. Symptoms of orange peel skin, nipple retraction or nipple discharge are uncommon. Unlike primary tumors, the skin is not involved and axillary lymph node involvement is variable. In our study, these were painless breast masses without associated axillary involvement [6].
Radiologically, these are circumscribed masses on mammography and hypoechoic masses on ultrasound with a predilection for the upper outer quadrant of the breast, as observed in our series. Calcifications and spiculation are rarely present, unlike primary breast carcinomas [7–9].
However, not all metastatic lesions present these characteristics and approximately one-third of the lesions do not present any particular histological characteristics [2].
Then, the patient’s clinical history is helpful in comparing pathologic features with the primary tumor. For example, as in our series, a clear cell carcinoma suggests a renal origin, a pleomorphic and mitotic spindle cell proliferation points towards a sarcoma.
In the absence of a medical history, immunohistochemical analysis is essential. In general, an antibody panel is necessary because no marker is 100% specific or sensitive. Anti-CK7 or anti-CK20 antibodies can guide the diagnosis. In men, primary breast cancers present almost a hormonal status and molecular identical to those of women in menopause. They are most often CK7 positive and CK 20 negative, associated with positive hormone receptors, while HER 2 is rarely positive. Thus, the positive expression of mammaglobin, RE, RP, GATA3 and GCDFP.15 helps to support the primitive mammary origin [1, 2, 10].
In the case of triple-negative carcinomas, the use of SOX-10 may be useful. The diagnosis of breast metastases will be confirmed by an immunohistochemical study combining the markers supporting the mammary origin and the expression of specific antigens of primary extra-mammary cancer (PAX8 and CD 10 for the kidney, PSA for the prostate ….) [11].
The management of breast metastases is essentially palliative, because of their poor prognosis by the frequent presence of localized metastases in other organs, like what we saw in our cases. Nevertheless, in the absence of other metastases detected, conservative surgical management was proposed with respect to healthy resection margins. Axillary dissection was not performed due to the clinical and radiological absence of satellite lymphadenopathy.
All this aims to emphasize the importance of suspecting the secondary origin, in order to avoid unnecessary mutilating surgery for patients, and to permit appropriate chemotherapy or radiotherapy.
CONCLUSION
In conclusion, breast metastases can clinically and radiologically mimic primary breast cancer. This reflects the importance of differential diagnosis, based on histology and supported by immunohistochemistry, with the help of the patient’s history when these are known, in order to avoid unnecessary mutilating surgery for patients and to offer them appropriate therapy.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
DATA AVAILABILITY
The data underlying this article will be shared on reasonable request to the corresponding author.



