-
PDF
- Split View
-
Views
-
Cite
Cite
Tai Hato, Masatoshi Yamaguchi, Ato Sugiyama, Kohei Aoki, Hiroki Fukuda, Mitsutomo Kohno, Mitsuo Nakayama, A case of cerebral infarction due to aplastic or twig-like middle cerebral artery after lung cancer surgery, Journal of Surgical Case Reports, Volume 2022, Issue 9, September 2022, rjac430, https://doi.org/10.1093/jscr/rjac430
Close - Share Icon Share
Abstract
Aplastic/twig-like middle cerebral artery is a rare vascular abnormality. We report a case of postoperative cerebral infarction caused by this disease. The patient is a male in his 40s. A 9-cm tumour was revealed to have invaded the superior vena cava from his right lung. He underwent right upper and middle bilobectomy. Due to the vascular invasion, the intraoperative bleeding exceeded 2 litres. Mechanical ventilation was required for postoperative pneumonia. After extubation, he was unable to write and was found to have cerebral infiltration in the left middle cerebral artery region. The cause of the cerebral infarction was investigated, but no thrombus in the left atrium or arteriosclerosis was found. No atrial fibrillation was observed during or after the surgery. Magnetic resonance angiography of the brain revealed an aplastic/twig-like middle cerebral artery.
INTRODUCTION
Cerebral infarction occurs in approximately 0.2–0.8% of patients after lung cancer surgery [1]. Thrombotic cerebral infarction is the most frequent cause. Known risk factors for post-lung-resection cerebral infarction include ageing, male sex, the presence of comorbidities, anticoagulant therapy and left upper lobectomy [2]. Here, we report an unusual case of postoperative cerebral infarction caused by a structural abnormality of the middle cerebral artery.
CASE REPORT
The patient was a male in his 40s. He visited an orthopaedic practitioner for multiple joint pain. No arthritis or rash was observed, but a tumour was detected on his chest X-ray, so he was referred to us (Fig. 1). He was a current smoker with a smoking index of 600. He did not have a past medical history other than chronic obstructive pulmonary disease. His mother suffered from lung cancer. He had an allergy to dairy products. A 9-cm irregular-shaped tumour was revealed in the hilum of the right lung by computed tomography (Fig. 2). The tumour seemed to invade the superior vena cava and azygos vein. Transbronchial biopsy was unsuccessful. The tumour was believed to be a non-small-cell lung cancer of clinical stage IIIB. As the tumour grew quickly, prompt surgery was scheduled.
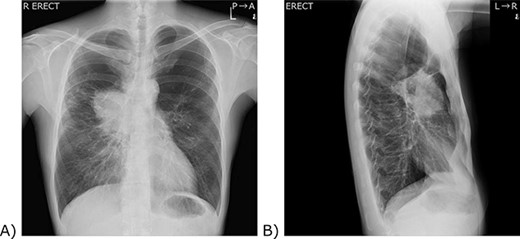
Chest X-ray. (A) A frontal view. (B) A lateral view. (A) A 9 cm tumour was located in the right hilum of the patient. The right pulmonary artery and the lower part of the superior vena cava are silhouetted out, and the boundary cannot be seen. (B) The tumour was located anterior to the hilum of the lung.
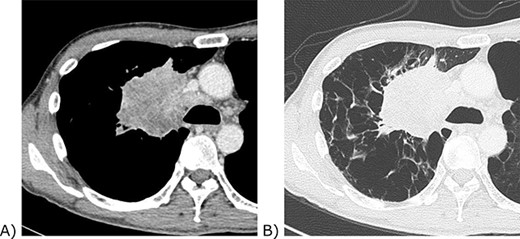
Chest computed tomography of the tumour. (A) Contrast-enhanced mediastinal window view. The tumour invaded the confluence of the superior vena cava and azygos vein. (B) Lung window view. The background lung was emphysematous.
A right upper and middle bilobectomy with mediastinal lymph node dissection was planned. The tumour firmly infiltrated at the confluence of the superior vena cava and the azygos vein. During exposure of the invaded area, the blood loss became excessive. A total clamp of the superior vena cava was placed, and a temporary bypass from the left brachiocephalic vein to the right atrium was established. The invaded vessel wall of the superior vena cava was removed and closed promptly. The operation time was 464 minutes. The total clamping time of the superior vena cava was 15 minutes. The amount of blood lost was 2384 g.
The patient required reintubation due to postoperative pneumonia and was extubated on day five. On that day, the patient complained of numbness in the little finger of his right hand, but no other neurological abnormalities were observed. The numbness was believed to be caused by nerve compression due to the patient’s posture, so the patient was followed up. On the ninth day, he was unable to write. Apraxia, such as writing and calculation, was observed, but no other symptoms were obvious. Brain magnetic resonance imaging revealed a cerebral infarction in his left parietal lobe (Fig. 3). Argatroban hydrate was administered, and the cause of cerebral infarction was investigated. No atrial fibrillation was detected during or after the surgery. Transthoracic echocardiography did not reveal a thrombus in the atrium or at the pulmonary vein stump. The computed tomography scan showed no cause of embolism on either side of the internal carotid arteries or the aortic arch. Magnetic resonance angiography showed hypoplasia of the origin of the middle cerebral artery and compensatory vascular formation (Fig. 4). The infarction was diagnosed as caused by an aplastic/twig-like middle cerebral artery (Ap/twig-like MCA). While undergoing rehabilitation, the patient completed postoperative adjuvant chemoradiotherapy after his discharge. He succeeded in returning to work six months after surgery. He has been alive without recurrence for eighteen months.
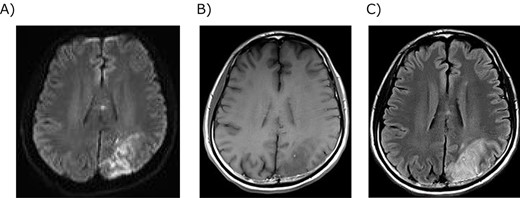
Magnetic resonance imaging of the brain infarction. (A) Diffusion-weighted image showed strong signals in the left parietal lobe. (B) T1-weighted image showed a low signal in the infarction. (C) T2-weighted image showed a high signal in the infarction.
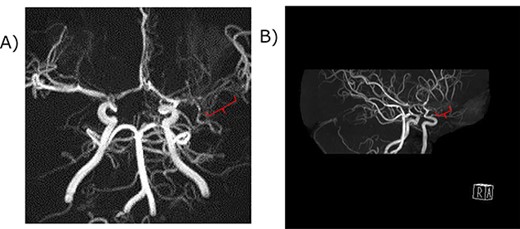
Magnetic resonance angiography of the middle cerebral artery. (A) A coronal view. (B) A Right anterior-lateral view. The red curly bracket indicates the immature M1 lesion of the middle cerebral artery. The distal part of the left middle cerebral artery was not atrophic, suggesting Ap/twig-like MCA.
DISCUSSION
Ap/twig-like MCA is a rare structural abnormality of the horizontal part of the middle cerebral artery [3]. The incidence is reported to be approximately 0.11–1.17% [4, 5]. This disease has been reported under various names, such as embryonic unfused MCA, twig-like MCA and unfused/twig-like MCA, creating a confusion in terminology [3–8]. The middle cerebral artery is formed by the fusion of reticulated vascular networks in utero [3]. It has been reported that this disease is caused by unknown factors hindering fusion in the M1 region and that a small network of vessels remains only in the horizontal region [3, 8].
The diagnosis of Ap/twig-like MCA is based on angiographic imaging. Differentiation from moyamoya disease is particularly important for the diagnosis [3, 6, 8]. Unlike moyamoya disease, Ap/twig-like MCA is unilateral [3]. Twig-like vessels are found only in the M1 region, and the vessel diameter after M2 is normal [3, 6]. Furthermore, Ap/twig-like MCA does not show collateral circulation from the external carotid artery, as seen in moyamoya disease [3, 6]. This disease does not progress. It is quite difficult to distinguish between unilateral moyamoya disease and Ap/twig-like MCA [6]. It is also important to exclude steno-occlusive diseases such as arteriosclerosis [3, 6].
Half of the cases of Ap/twig-like MCA are found in intracerebral haemorrhage [6]. Approximately, 20% of cases are found because of infarction [6, 7]. It has been reported that an aneurysm is easily formed because the blood vessel wall is fragile in Ap/twig-like MCA. Shin et al. reported that the wall of the middle cerebral artery observed intraoperatively was highly hypoplastic in Ap/twig-like MCA [9]. Due to this fragility, flow-related aneurysm formation was stimulated, and rupture could occur. Revascularization may be a treatment of choice [7].
In this case, it was difficult to detect cerebral infarction due to the clinical course of long sedation due to pneumonia and localized neurological symptoms. No thrombus was confirmed by examination after the detection of cerebral infarction, and the clinical picture did not match the typical postoperative cerebral infarction in lung cancer. Since abnormalities in the cerebrovascular disease were confirmed, it was speculated that this case was a cerebral infarction caused by Ap/twig-like MCA triggered by hypotension due to intraoperative haemorrhage. No reports of Ap/twig-like MCA causing cerebral infarction associated with extracranial surgery could be found within the scope of the investigation. However, it has been reported that moyamoya disease, which also exhibits dysplasia of the middle cerebral artery, causes cerebral infarction after extracranial surgery [10]. In addition, since the localization of cerebral infarction occurred only in a limited area of the MCA region, it is highly possible that Ap/twig-like MCA was the cause of the cerebral infarction in this case.
CONCLUSION
Abnormalities in cerebrovascular structure are rare but should be noted as a potential cause of postoperative cerebral infarction.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to declare.
FUNDING
None.
CONSENT
Informed consent was obtained from the patient.
GUARANTOR
Tai Hato.
References
- atrial fibrillation
- arteriosclerosis
- left atrium
- magnetic resonance angiography
- cerebral infarction
- superior vena cava
- middle cerebral artery
- surgical procedures, operative
- brain
- neoplasms
- surgery specialty
- thrombus
- extubation
- mechanical ventilation
- intraoperative hemorrhage
- right lung
- postoperative pneumonia
- lung cancer surgery



