-
PDF
- Split View
-
Views
-
Cite
Cite
Foteini Karasavvidou, Lampros Mitrakas, Styliani Tzika, Ioannis Zachos, Athanasios Anagnostou, Vassilios Tzortzis, Mixed epithelial and stromal tumor—adult cystic nephroma of the kidney: a case report with immunohistochemical analysis, Journal of Surgical Case Reports, Volume 2022, Issue 9, September 2022, rjac387, https://doi.org/10.1093/jscr/rjac387
Close - Share Icon Share
Abstract
The mixed epithelial and stromal tumor family of kidney contain neoplasms with biphasic epithelial and stromal component. According to the 2016 World Health Organization Classification, they encompasses a spectrum of tumors ranging from predominantly cystic tumors (adult cystic nephroma) to tumors that are variably solid (Mixed epithelial and stromal tumor-MESTs). We present the case of a 20-year-old woman with an adult cystic nephroma which was verified by immunohistochemical examination.
INTRODUCTION
Mixed epithelial and stromal tumors of the kidney (MEST) are rare, accounting for 0.2% of all renal tumors, and distinctive neoplasms with biphasic epithelial and stromal component. According to the 2016 World Health Organization (WHO) Classification, they encompass a spectrum of tumors ranging from predominantly cystic tumors (adult cystic nephroma/ACN) to tumors that are variably solid (MEST) [1].
ACN was previously classified as paediatric cystic nephroma, a separate entity from MEST. However, on the basis of similar age and sex distributions, as well as similar molecular, immunohistochemical profile and overlapping histological features, adult cystic nephroma is now classified within MEST family [2, 3].
Hereby, we present the first case of MEST in Greece.
CASE REPORT
A 20-year-old Caucasian woman presented herself to the outpatient department because of a mass on the left kidney. The mass was an incidental finding in an abdomen computed tomography (CT), which was suggested within the diagnostic evaluation for a persistent flank pain. In the past, she never had any pathologic urologic situation. According to the CT finding, the mass was located on the lower pole of the left kidney (83 × 111 × 111mm) and there was no other pathologic finding. Anamnestically, she had heterozygous beta Mediterranean anaemia. The patient was obese (body mass index = 33.4 kg/m2). We proceeded with a thorax CT which revealed no pathology. The patient underwent a laparoscopic nephrectomy by an urologist well trained and experienced in laparoscopy. Macroscopically, a mass in the lower pole of the left kidney (110 × 100 × 100 mm) was documented. Cut sections revealed multiple thin walled, non-communicating cysts of varying sizes with smooth lining without solid component. Microscopically, the tumor was characterized of cysts separated by thin septa. The cysts lined by single layer of flat, cuboidal and hobnail epithelium and the septa were fibrous, hypocellular to hypercellular. No mitoses or necrosis were identified. The immunohistochemical examination showed that the epithelial cells were positive for the cytokeratins AE1/AE3 and PAX-8. The stromal cells were positive for the progesterone receptors, estrogen receptors and CD10. The latter was concentrated around epithelial elements (Figs 1 and 2). These findings established the diagnosis of ACN. At the 12-month follow-up control with abdomen CT, there was no pathologic finding (Fig. 3). The patient is under urologic and nephrologic montitoring without any abnormal finding at 21 months after surgery. This research complies with the guidelines for human studies and was conducted ethically in accordance with the World Medical Association Declaration of Helsinki.
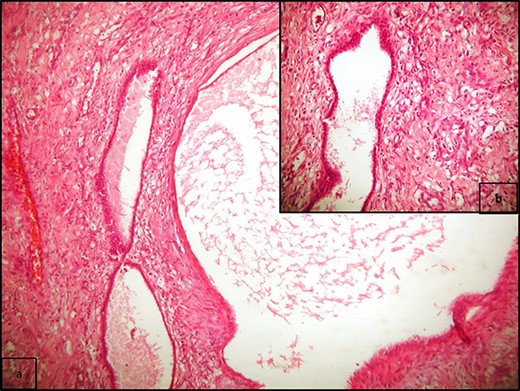
(a) Multiple cysts of varying sizes separated by septa; flat epithelial cells lining the cysts, and there is no atypia or mitosis (H/E x400); (b) cysts separated by hypercellular stroma (H/E x100).
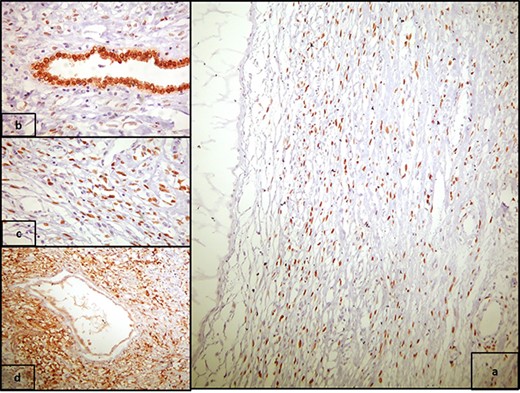
(a) PR positivity in the stromal spindle cells (PR x400), and (b) strong nuclear PAX8 positivity in epithelial cells (PAX8 x100); (c) high power view of 2a (PR x100); (d) CD10 positivity in the stromal spindle cells, concentrated around epithelial elements (CD10 x100).
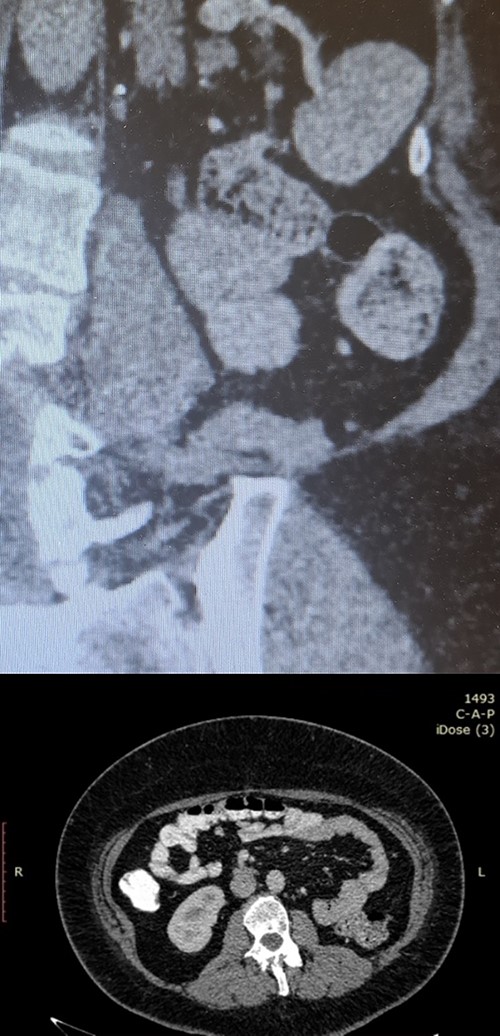
Postoperative follow-up imaging with abdomen CT at 12 months; coronal (upper side) and axial (lower side) planes, and no evidence of local recurrence, and no other pathologic finding.
DISCUSSION
The MEST family contains neoplasms with biphasic epithelial and stromal component with spindle stroma, glands and cysts. They are uncommon renal tumors first described by Michal and Syrucek in 1998 and were subsequently included in the WHO classification of tumors in 2002 [4, 5]. ACN and MEST were considered as separate entities in the 2004 WHO classification of renal neoplasms [6]. Recent studies suggested that ACN and MEST share clinicopathologic features and may represent the same disease process of varying morphology. According to the 2016 WHO Classification, they encompass a spectrum of tumors ranging from predominantly cystic tumors (ACN) to tumors that are variably solid (MESTs). Paediatric cystic nephroma is a distinct entity [1].
MESTs typically affect perimenopausal women and the mean patient age is 52 years. In males, there is often a history of hormone therapy such as androgen deprivation or oestrogen therapy for prostate cancer [7]. Mostly, these tumors occur with flank pain and haematuria. On imaging, MEST appears as a well-circumscribed multiseptate cystic mass with thin or thick septae and enhancing solid components and is usually characterized as a Bosniak III or IV cyst. A pure solid pattern has also been reported [8]. A pre-operative diagnosis of MEST cannot be made based on imaging studies.
Consequently, these patients are treated with radical or partial nephrectomy. They are solitary, rarely bilateral, unencapsulated but well circumscribed [9]. The mean size is 9 cm. They usually involve the medulla or they may be within the renal pelvis. Taking into account these features (size, involvement of medulla or invasion of renal pelvis), a nephron-sparing surgery is not usually feasible. On the other hand, partial nephrectomy remains an option for patients with cT1 as well as cT2 tumors and a solitary kidney or chronic kidney disease. Cut surface reveals variable proportions of solid and cystic areas. The cysts are of different sizes and shape with smooth and glistening linings and contain serous fluid [10]. Microscopically, the cysts are lined by flat, cuboidal, hobnail, columnar cells with eosinophilic, amphophilic or vacuolated cytoplasm, clear cells or ciliated cells. The epithelium may has Müllerian features. Rarely, it may be pyloric, intestinal or urothelial-like [3]. The stroma ranges from hypocellular to hypercellular. Ovarian-type stroma is often present and may undergo secondary luteinization. Stromal condensation may be around the epithelial component and rarely myxoid change can occur [3]. Atypia is usually minimal in both epithelial and stromal component. Smooth muscle, lipomatous metaplasia, mitosis, necrosis and haemorrhage are rare findings. Immunohistochemically, epithelium shows positivity for cytokeratins, PAX2 and PAX8 [11]. GATA3 co-expression with PAX8 has been found in 57% of the cases [12]. Stroma usually reveals strong and diffuse positivity for SMA, desmin, caldesmon, ER and PR [12]. CD10 is often concentrated around epithelial elements [12]. Inhibin, calretinin and FOXL2 are positive in ovarian type stroma, with inhibin and calretinin positivity particularly in luteinized cells [13]. The vast majority of MEST is benign and has a good prognosis. Malignant transformation is reported and indicates a poor prognosis [14]. Only 2 cases of recurrence are reported [15]: (i) a late recurrence after a primary incomplete surgical resection, which was excised with a portion of adherent liver and (ii) an early recurrence due to malignant transformation, which was treated with palliative radiotherapy and chemotherapy.
In summary, MEST is a rare clinical entity. It must be suspected in a patient presenting with a complex cystic renal mass particularly if this appears to be extended into the collecting system without evidence of local or distant tumor spread. These tumors are predominantly benign, but malignant transformation is reported. Tumor excision with clear margins is the standard of care.



