-
PDF
- Split View
-
Views
-
Cite
Cite
Abdullah Alkhayal, Khalid Alrabeeah, Bader H Alsaikhan, Abdulaziz Alathel, Fahad R Barayan, Abdullah Alsaghyir, Recurrent urethral stricture with dual lumen managed by a single stage dorsal Onlay buccal mucosal graft Urethroplasty: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 8, August 2022, rjac365, https://doi.org/10.1093/jscr/rjac365
Close - Share Icon Share
Abstract
Urethral stricture is defined as any abnormal narrowing throughout the entire length of the male urethra. Anterior urethral stricture is the most common site which accounts for more than 90% of cases in developed countries. One of the possible long-term outcomes of urethral stricture treatment is stricture recurrence. Refractory urethral strictures to initial management present surgical challenges to the reconstructive surgeon. It has been reported in the literature that buccal mucosal grafts in complex anterior urethral strictures have lately become a well-established management modality for bulbar and penile urethral strictures. Here, we are presenting an interesting case of a patient with a dual lumen urethra due to recurrent urethral stricture.
INTRODUCTION
Urethral stricture is defined as any abnormal narrowing throughout the entire length of the male urethra [1]. Anterior urethral stricture is the most common site which accounts for more than 90% of cases in developed countries. Urethral stricture can be caused by multiple etiologies [1] . The most common etiologies are trauma, infection and iatrogenic or surgical interventions [2]. The prevalence of male urethral stricture is ~0.6% in western countries [3]. Presentation of a patient with urethral stricture ranging from mild to severe obstructive lower urinary tract symptoms [4]. Including hesitancy, weak stream, incomplete emptying, post void dribbling, painful or less forceful ejaculation, hematuria and urinary retention can lead to renal function deterioration [1, 4]. The evaluation of patients suspected to have a urethral stricture includes history, physical examination, uroflowmetry and imaging including antegrade and retrograde urethrogram (RUG) or voiding cystourethrogram (VCUG) and cystoscopy [5]. Urethral strictures can be treated surgically using two main approaches, endoscopic management such as Dilatation (Direct Vision Internal Urethrotomy [DVIU]) and urethroplasty [5]. One of the possible long-term outcomes of urethral stricture treatment is stricture recurrence. Here, we are presenting an interesting case of a patient with a dual lumen urethra due to recurrent urethral stricture.
CASE PRESENTATION
A 38-year-old male was referred to our tertiary hospital due to recurrent urethral stricture for further evaluation and management. His history revealed multiple failed attempts of DVIU, anastomotic urethroplasty and urethroplasty with pedicled fasciocutaneous flap in 2019. Nine months after his surgery, he had complete retention due to stricture recurrence and he underwent multiple dilatations and DVIU but his stricture keep recurring. He was referred to us for definitive management. He was on a suprapubic catheter. Initial workup included retrograde, antegrade cystoscopy and urethrogram. First, an antegrade cystoscope was introduced. The bladder neck, internal sphincter and verumontanum were identified. However, there was no external sphincter due to damage from the previous procedures. A pinpoint opening in the dorsal area of the urethra was seen about 1-cm distal to the verumontanum (Fig. 1, lumen A). Also, a blind-ended urethra in the ventral area was identified (Fig. 2, lumen B). It is most likely from the previous flap. Additionally, the retrograde urethrogram showed that the contrast was only going from the pinpoint hole in the dorsal side of the urethra Fig. 3. Retrograde cystoscopy also showed a blind-ended in the proximal bulbar urethra. We could not identify the small hole that we saw from the antegrade cystoscopy. After initial workup, he was counseled in the clinic for dorsal only buccal mucosal graft (BMG) urethroplasty for which he agreed.
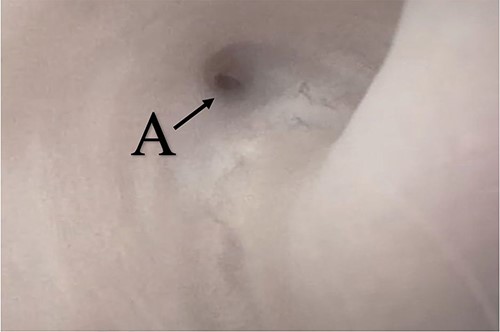
(lumen A): A pinpoint opening in the dorsal area of the urethra as pointed in the arrow.
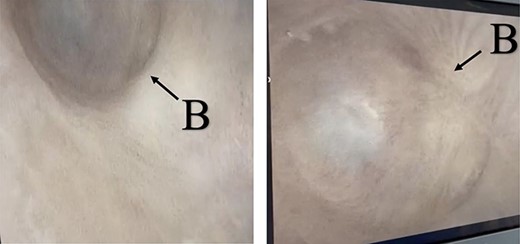
(lumen B): A blind-ended urethra in the ventral area of the urethra as pointed in the arrow.
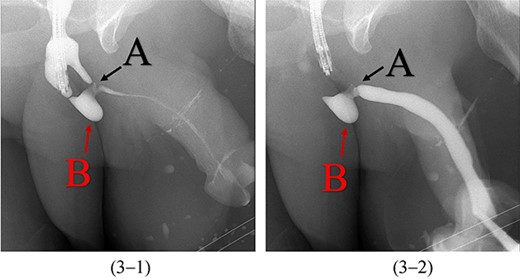
Antegrade (3–1) and retrograde (3–2) urethrogram showing the contrast passing through the pinpoint hole in the dorsal side of the urethra as pointed in black arrow, lumen A; antegrade (3–1) and retrograde (3–2) urethrogram showing a blind ended urethra in the ventral side of the urethra as pointed in red arrow, lumen B.
In the beginning, an inverted Y midline perineal skin incision was made extending from the base of the scrotum to just above the anus. There was an extensive adhesion reaching up to the Colle’s fascia and they were incised. Then, a cystoscope was introduced through the suprapubic channels and a guidewire was inserted through the pinpoint stricture. An 18-French Red Rubber Catheter was advanced per urethral meatus to the stricture. The urethra was dissected from the corpora cavernosa and rotated. After that, the urethra was incised dorsally over the distal tip of the catheter to open lumen A over the previously inserted guidewire (Fig. 1, lumen A). Once lumen A was completely open, the other channel was ventral to the scarred urethral plate. Therefore, the decision was to completely excise this channel to leave the ventral service lined by the previously placed fasciocautonous flap. The resected area is marked by red line in Fig. 4. Then, refashioning the proximal part and distal flap to form the ventral covering of the urethra. After that, harvesting the BMG was done. The harvested BMG was tailored and placed dorsally where it was split-fixed to the corporal body. The proximal and distal apical edges of the graft were sutured to the corresponding dorsal wall of the corpus spongiosum using an interrupted full-thickness 5–0 PDS. After that, the lateral edge of the corpus spongiosum was secured to the lateral aspect of the graft and the underlying tunica albuginea of the corpora using a 5–0 PDS suture on each side allowing the flap to rotate to its original position. Before completion, a 16-French urethral catheter was advanced easily into the bladder. Subcutaneous tissues that included a part of the bulbous spongiosum muscle and Colle’s fascia were closed in a continuous fashion using 3–0 Vicrl. Finally, the skin was closed using 4–0 Vicry-Rapide. The postoperative course was uneventful. Four days later, he was discharged home in a healthy condition. The patient recovered from surgery.
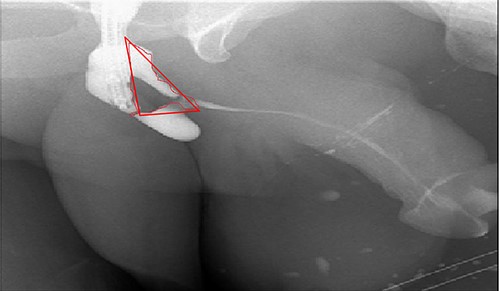
Antegrade urethrogram with the resected area marked in red line.
A retrograde urethrogram was performed after 4 weeks which revealed no leakage, and a patent urethra with a wide anastomotic site at the bulbar urethra Fig. 5. Urethroscopy was also performed, and it confirmed an easy passage with no stricture recurrence. Therefore, the urethral catheter was removed. The uroflowmetry showed an excellent Q max 29 ml/s and voided 378 ml with minimal post voiding residual 30 ml/s. The patient was happy with the results.
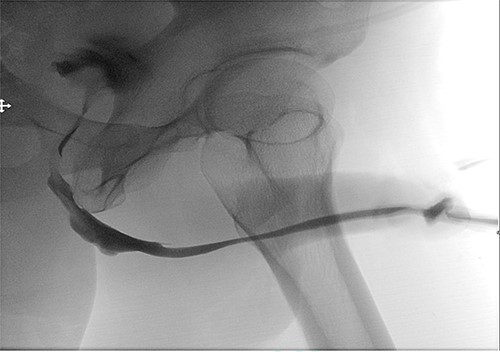
Postoperative retrograde urethrogram revealing no leakage, and a patent urethra with a wide anastomotic site at the bulbar urethra.
DISCUSSION
Refractory urethral strictures to initial management present surgical challenges to the reconstructive surgeon because it is considered a complex stricture that requires tissue transfer or staged urethroplasty [6, 7]. It has been reported in the literature that BMGs in complex anterior urethral strictures have lately become a well-established management modality for bulbar and penile urethral strictures compared with penile fasciocutaneous flap and scrotal skin [6]. Especially for those who are not suitable for anastomosis or excision [6]. Furthermore, the BMG has an advantage over other tissues as it resembles urethral mucosa and is easily harvested [7]. In addition, it has been found that repeating endoscopic management such as dilatation and DVIU is not beneficial in a complex resistant stricture [7].
Redo BMG urethroplasty represents the best evidence base as a graft source for those who had complex recurrent strictures. A study was conducted by Javani D et al. that aimed to evaluate the outcome of redo BMG urethroplasty in patients presenting with recurrent anterior urethral stricture. They have included 21 patients who underwent redo BMG urethroplasty and were followed up to 6 years with a success rate of 85.7% [8]. In addition, another study has taken place in Germany aimed to determine the success rate, oral morbidity and functional outcomes of redo BMG urethroplasty. The success rate of redo BMG urethroplasty was 82% and recommended that redo BMG urethroplasty is an appropriate procedure and therapeutic option for complex recurrent strictures [9].
In the present case, our patient had a dual lumen urethra after failed fasciocautonous flap placement. This case was managed successfully with a single stage BMG urethroplasty. Five weeks postoperatively, RUG was done, and it showed an excellent result with a patent urethra and a wide anastomotic site at the bulbar urethra.
CONCLUSION
Failed urethroplasty could lead to complex strictures that can be managed by a redo BMG urethroplasty. To the best of our knowledge, this is the first case that reports such a rare finding of a dual lumen urethra after failed fasciocautonous flap urethroplasty. In the present case, the BMG showed an excellent outcome with no postoperative complication.



