-
PDF
- Split View
-
Views
-
Cite
Cite
Carolina Arró Ortiz, Darío Ramallo, Nicolás Guerrini, What is the ideal management of Krukenberg syndrome?, Journal of Surgical Case Reports, Volume 2022, Issue 7, July 2022, rjac328, https://doi.org/10.1093/jscr/rjac328
Close - Share Icon Share
Abstract
We present the case of a 34-year-old female patient diagnosed with Krukenberg Syndrome, in which we performed total cytoreduction surgery of the lesions, with subsequent perioperative chemotherapy. After a follow-up of three years, we observe she continues without evidence of disease. In the early 1990’s Sugarbaker et al. introduced cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) as a new innovative therapy option for selected patients with peritoneal carcinomatosis. Nowadays, there is no established treatment for patients with peritoneal metastasis of colorectal cancer. There is a need for the future high-quality randomized multicenter trials to make a strong recommendation.
INTRODUCTION
Peritoneal metastasis of colorectal cancer (CRC) is the fourth most common recurrence site in all CRC patients, occurring in about 10% of these patients. This is related to the poor prognosis of the patient and the 5-year survival rate is reported to be about 20–30% despite treatment with chemotherapy [1].
Krukenberg tumor is defined as a tumor of ovarian location with characteristic histology, which is an infrequent tumor, has high morbidity and mortality. About 90% of cases correspond to metastases from primary gastric and colon neoplasms.
Herein, we report a case of patient developing peritoneal metastasis of colorectal cancer.
CASE REPORT
A 34-year-old female patient with a history of Hartmann’s operation for occlusive acute abdomen three years previously. The pathological examination confirmed a sigmoid colon, well-differentiated panmural adenocarcinoma, without vasculolymphatic invasion and with perineural permeation (High-risk stage II-pT4b N0 Mx).
The patient received eight cycles of chemotherapy treatment with Capecitabine and Oxaliplatin for six months. One month after the adjuvant; thorax, abdomen and pelvis CT scan detected a cystic image in the right ovary. The transvaginal gynecological ultrasonography was normal. The colonoscopy did not show pathological findings. The serological test reported normal levels of the carbohydrate antigen 19-9 (CA 19-9) of 13.5 UI/ml (reference range: 0–37 UI/ml) and a slight increase of the carcinoembryonic antigen (CEA) of 5.5 ng/ml (reference range: 0–4.7 ng/ml). The clinical and imaged follow-up showed the stability of the disease; after four months, the Department of Surgery performed the intestinal reconstruction laparoscopic surgery.
In successive controls, laboratory results revealed elevated levels of serum CEA of 28.2 ng/ml. Magnetic resonance (MR) detected adenopathy in aortoiliac bifurcation, 18 × 8 mm of undetermined origin, and a lesion in the right adnexal, 36-mm witch could correspond to a neoplasia process linked to endometrioma (Fig. 1). PET-CT scan informed adenopathy in aortoiliac bifurcation (SUV 7.3) and an image in the right adnexa with a mixed component (SUV 23) (Fig. 2).
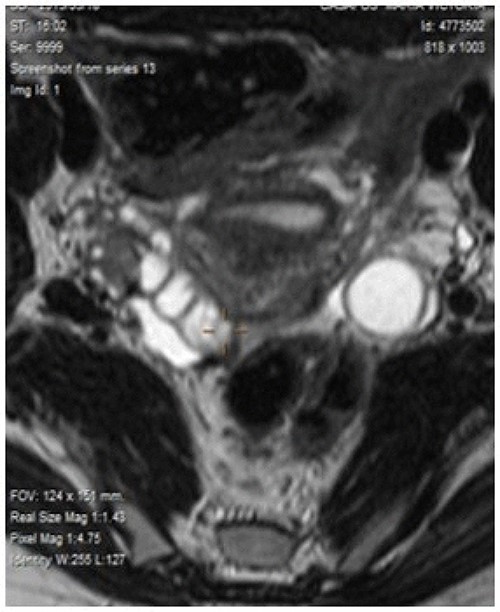
Magnetic resonance detected adenopathy in the aortoiliac bifurcation of undetermined origin, and a lesion in the right annex, which could correspond to a neoplastic process linked to endometrioma of the right ovary or to a tubal origin.
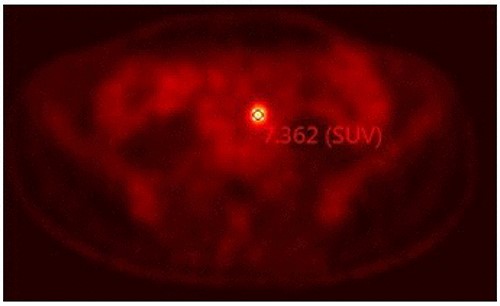
PET-CT scan informed adenopathy in the aortoiliac bifurcation (SUV 7.3).
On multidisciplinary discussion, a decision of laparotomy surgery was performed. Right salpingo-oophorectomy and adenopathy resection were performed. Pathological gross examination during the surgery revealed a tumor infiltration of possible intestinal origin and a necrotic process without evidence of tumor infiltration for the adenopathy.
Postoperative pathology confirmed that the right ovarian parenchyma had an infiltration by carcinoma of the intestinal type, with corpus luteum and endometriosis; and material sent as a ganglion corresponds to a giant cellular reaction by foreign material. Immunohistochemistry: CK7, CA 125 and WT1 were negative (Fig. 3), CK20 was positive, intense and diffuse in metastatic proliferation (Fig. 4). The histopathologic confirmed a Krukenberg tumor of the ovary and immune profile favored a metastatic gastrointestinal carcinoma.
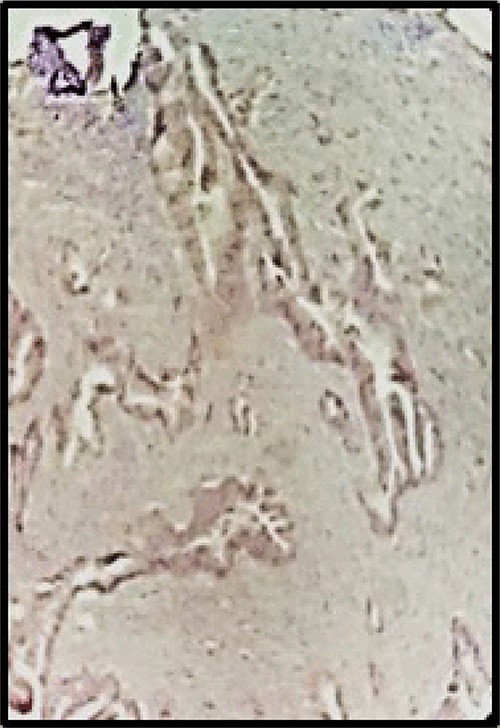
Immunohistochemistry: CK7, CA 125 and WT1 were negative, (HE 40X).
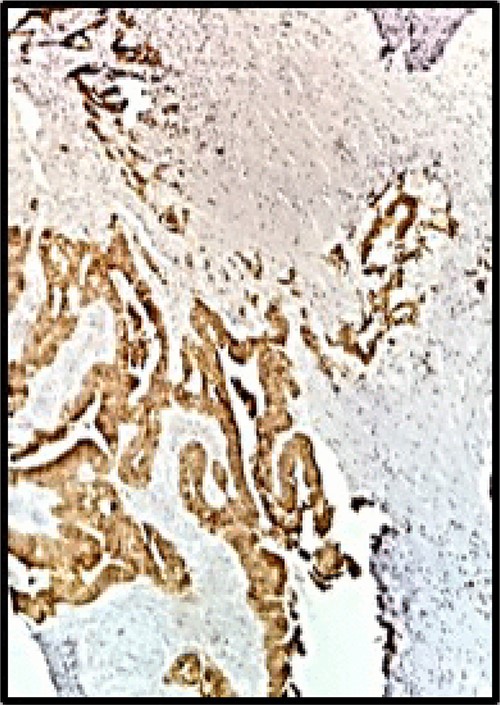
Immunohistochemistry: CK20 was positive, intense and diffuse (HE 40X).
The postoperative outcome was uneventful. The patient completes the chemotherapy treatment: eight cycles with Capecitabine and Oxaliplatin. Currently, three years after the initial diagnosis, she is in clinical follow-up with a favorable outcome, and no evidence of recurrence.
DISCUSSION
While the concept of cytoreduction and metastasectomy is accepted in the management of CRC with solid organ metastases, optimal management of CRS with peritoneal metastases (PM) remains controversial. A large retrospective multicenter study of 506 patients from 28 institutions found that patients with PM from CRC who underwent complete CRS (and perioperative chemotherapy) had a median survival of 32.4 months compared with 8.4 months in patients in whom a complete cytoreduction was not possible [2]. Studies from the early 2000s demonstrated a survival benefit for patients undergoing CRS with HIPEC for CRC with PM compared to systemic chemotherapy (Fluorouracil [5-FU]/Leucovorin) [3]. Several studies have reported that the 5-year survival rate of patients undergoing this treatment is over 45%. However, CRS and HIPEC are technically challenging, have limitations and require a well-trained team, with high postoperative complications and a long learning curve compared to other surgeries.
Results from the PRODIGE 7 provide an assessment of the role of CRS and HIPEC in the management of CRC with PM. In this randomized phase III multicenter trial, 133 patients were treated with either CRS and HIPEC (intraperitoneal Oxaliplatin, intravenous 5-FU/Leucovorin) and 132 patients were treated with CRS alone. After a median follow-up of 63.8 months, the median overall survival was not significantly different between the arms. Major complications were increased in the CRS/HIPEC arm (24.1 vs 13.6% in the other group) [4]. The median recurrence-free survival was 13.1 months in HIPEC arm and 11.1 months in non-HIPEC arm (P = 0.486). The overall postoperative mortality rate was 1.5% in both groups [4].
The sentinel prospective trial by Verwaal et al. randomized a cohort of 105 patients to systemic chemotherapy (5-FU and leucovorin) with or without palliative surgery vs CRS/HIPEC. At a median follow-up of 21.6 months, the overall median survival was 22.3 months in CRS/HIPEC patients vs 12.6 months in the control arm. In patients with complete macroscopic cytoreduction (CCR-0/1), median survival was 48 months and a 5-year survival rate was 45%, respectively. The survival differences favoring CRS/HIPEC were still evident at 8-year follow-up: median progression-free survival was 12.6 vs 7.7 months, and the median disease-specific survival was 22.2 vs 12.6 months. An often-discussed limitation of this trial is that the systemic chemotherapy arm only included 5-FU/Leucovorin, which is not an optimal regimen by today’s practices. It should also be noted that 18 (17%) patients in the total cohort had primary tumors arising from the appendix. The Swedish peritoneal study (randomized phase III trial) concluded that CRS with HIPEC may be superior to the systemic Oxaliplatin-based treatment of CRC with resectable isolate peritoneal metastases (median overall survival: 25 months vs 18 months, P = 0.04). [5].
Several preliminary studies showed a 5-year overall survival of up to 40% in those patients receiving CRS and HIPEC; however, there is a lack of studies of better quality to support this therapy. Besides, it should be taken into account that in our work environment, HIPEC therapy costs high. In this context, we present this clinical case as a therapeutic option for these patients.
CONFLICT OF INTEREST STATEMENT
All authors declare no personal, professional or financial conflicts of interest.
Patient consent was obtained for publication of the case details.
FUNDING
No funding/financial support was present in this case report.



