-
PDF
- Split View
-
Views
-
Cite
Cite
Lina Cadili, Krystal L Cullen, Nicola J Finn, Andrew Singh, Eric Webber, Allen H Hayashi, A rare case of a congenital pancreatic duplication cyst in an infant complicated by an upper GI bleed, pancreatitis, cyst infection and gastric outlet obstruction, Journal of Surgical Case Reports, Volume 2022, Issue 7, July 2022, rjac326, https://doi.org/10.1093/jscr/rjac326
Close - Share Icon Share
Abstract
Enteric duplication cysts are rare congenital entities most commonly found in the esophagus, ileum or colon but can be in remote locations such as the biliary tree, liver or pancreas. Pancreatic duplication cysts are very uncommon and usually present in adulthood with pancreatitis or abdominal pain. Here, we present a unique and complex case of an infant with a pancreatic duplication cyst initially presenting with an upper gastrointestinal bleed followed by pancreatitis, cyst infection and gastric outlet obstruction.
INTRODUCTION
Gastrointestinal (GI) tract duplication cysts are rare congenital malformations that can occur anywhere in the alimentary tract or adjacent organs [1, 2]. The incidence is 1 in 4500 births, with a slight male predominance [3]. The cysts are usually unilocular, lined by GI epithelium and surrounded by smooth muscle [4]. They are mostly found in the esophagus, ileum and colon [1]. Very rarely can these cysts be found in other locations such as the biliary tree, liver, pancreas or even the tongue [2]. Usually, at these locations they do not communicate with the parent GI tract and their blood supply is derived from the organ with which they are associated [2]. Pancreatic involvement is extremely rare, with only 57 cases documented currently [2, 4]. These patients typically present in adulthood with pancreatitis and abdominal pain.
The cause of enteric duplication cysts is not well understood but has been attributed to environmental stress affecting early fetal development [2]. Pancreatic duplication cysts are even less understood due to the shared pancreatic blood supply and theorized association with the pancreatic duct. Spivak et al. [2] postulate that the connection between the cyst and pancreatic duct is a result of inflammation and ulceration within the cyst that may perforate into a duct of the adjacent pancreas creating an internal fistula. Duodenal and pancreatic duplication cysts lead to unique treatment issues given their surrounding anatomy; however, they should not be ignored due to their possible long-term risk of malignant transformation. Here, we present a rare and unique case of an infant with a pancreatic duplication cyst who presented initially with an upper GI bleed, followed by pancreatitis, cyst infection and gastric outlet obstruction (GOO).
CASE REPORT
The patient is an 8-month-old otherwise healthy female who presented at 4 months of age with an upper GI bleed. An esophagogastroduodenoscopy revealed a peptic ulcer in D1 with no active bleeding. She was treated with proton pump inhibitors and imaging revealed a mass in the lesser sac with a broad differential including duodenal perforation into the lesser sac possibly from a foreign body, perforation of an upper GI duplication cyst or pancreatitis with pseudocyst formation (Fig. 1). Bloodwork was unremarkable other than elevated lipase at 177 units/L (N < 82 units/L). The presumed diagnosis was a post-pyloric duodenal ulcer and pancreatitis. The infant then re-presented to hospital with new non-bloody emesis and a repeat magnetic resonance imaging (MRI) revealed the same mass in the lesser sac. Further review of the imaging with specialists raised suspicion for a duodenal duplication cyst. The patient then developed signs of cyst infection and a rising WBC count (22.7 × 10^9/L) and was treated with antibiotics. Her vomiting progressed due to GOO secondary to extrinsic compression of the pre-pyloric antrum.
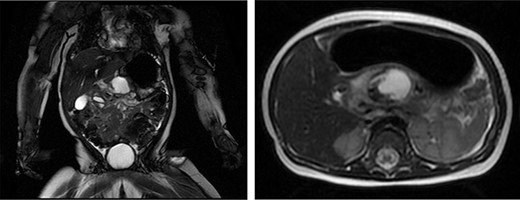
The patient underwent a radial endoscopic ultrasound which revealed a prepyloric, subepithelial cystic lesion with a mature wall consistent with a duplication cyst. Multidisciplinary discussions involving Pediatric Surgery, Pediatrics, Gastroenterology and Radiology raised several possible management strategies including medical management with antibiotics and nasogastric tube decompression, percutaneous cyst drainage, endoscopic cystgastrostomy with a metal stent or marsupialization and surgery in the form of cyst resection or drainage and cystgastrostomy. The group felt surgery would provide the most definitive treatment; thus, she was taken to the operating room for an exploratory laparotomy via a right subcostal incision. The small bowel from the ileocecal valve to the ligament of Treitz was normal with no evidence of other duplication cysts or Meckel’s diverticulum, and normal rotation.
We noted that the patient had an annular pancreas; there was pancreatic tissue around D2 with no evidence of stenosis. There were dense adhesions between the posterior wall of the stomach and this mass, which was essentially embedded within the head of the pancreas and closely positioned but separate from the medial wall of D1 and posterior stomach. There was scarred inflammation extending along the transverse mesocolon, but no true fistula was seen (Fig. 2). There was no evidence of a connection to the pancreatic duct. An intraoperative ultrasound revealed a thick-walled cyst; the fluid in the cyst was of low density with areas of mucous debris dependently (Fig. 3).
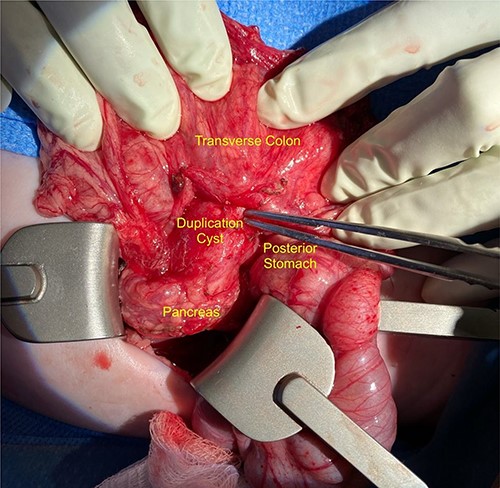
Intraoperative image showing the duplication cyst in relation to surrounding organs, and the inflammatory tissue extending from the mass to the colon (at the tip of the forceps).
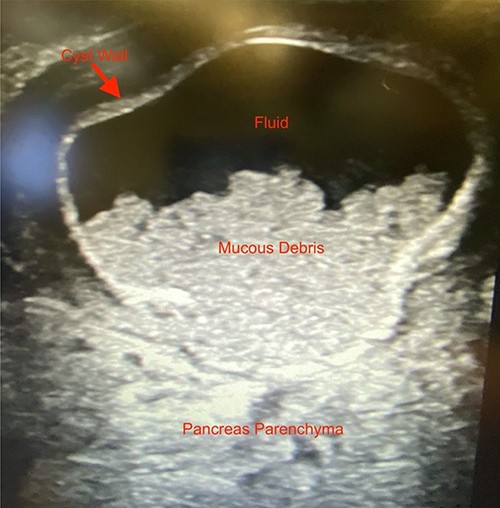
Intraoperative ultrasound image revealing a thick-walled mass intimately associated with the pancreas filled with fluid and mucous debris.
Given the location of the cyst, we felt resection would require a pancreaticoduodenectomy. Alternatively, because there was no biliary obstruction or evidence of chronic/recurring pancreatitis, it was safer to internalize the duplication cyst. The anterior cyst wall was opened, and its contents were about 2 ml of clear white fluid and a small amount of white mucous debris; samples were sent for culture and lipase, but unfortunately the amount of sample was not adequate for lipase. A mucosectomy (to mitigate the long-term risk of malignant transformation) and cystgastrostomy to the posterior antral wall were performed (Fig. 4). A Penrose drain was placed across the cystgastrostomy to maintain patency (Fig. 5).
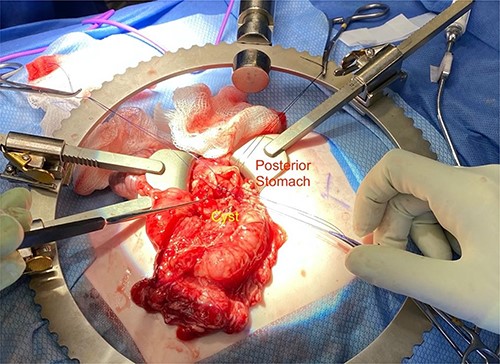
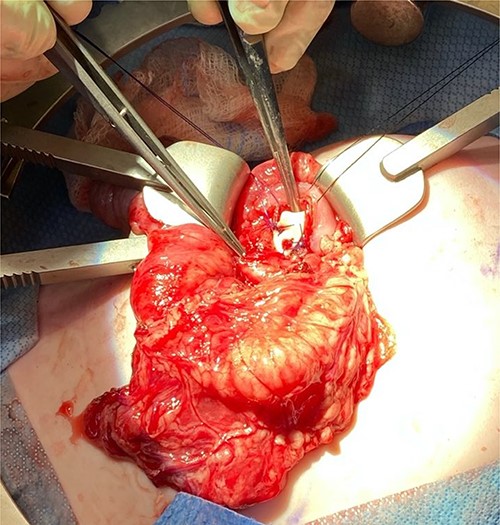
Penrose drain fixed across the cystgastrostomy with PDS sutures.
Postoperatively, the patient required nutritional support with total parenteral nutrition until gastric emptying was normal, and she was discharged home on a pureed diet on postoperative day 18 with a plan for endoscopic assessment in 8 weeks. The pathology from the mucosectomy revealed muscularis mucosa lined by gastroduodenal junctional mucosa.
DISCUSSION
Enteric duplication cysts are rare congenital entities with an incidence of 1 in 4500 births that can be found anywhere along and outside the GI tract [3]. Pancreatic duplication cysts are uncommon with few documented cases [2, 4]. Our case demonstrates a duplication cyst embedded within the pancreas in an infant who presented with pancreatitis, an upper GI bleed, cyst infection and GOO. Treatment options can include percutaneous or endoscopic drainage; however, these are temporizing measures, and these cysts ultimately require definitive treatment. Ideally, this would be in the form of resection, but pancreatic duplication cysts provide unique challenges for resection due to anatomic considerations. We performed mucosectomy and internal drainage in the form of a cystgastrostomy, to mitigate recurrence and long-term risk of malignant transformation. The finding of an annular pancreas perhaps suggests that pathogenesis could stem from embryologic development of the duodenum. This case illustrates the importance of a wide differential diagnosis in patients presenting with multiple problems and multidisciplinary collaboration for such cases.
CONFLICT OF INTEREST STATEMENT
None declared.



