-
PDF
- Split View
-
Views
-
Cite
Cite
Álex Morera-Grau, María Evangelina Patriarca-Amiano, Pablo Santiago-Díaz, Laia Serrano-Munné, Benedetto Ielpo, Fernando Burdío-Pinilla, Miguel Pera-Román, Silvia Espuelas-Malón, Mar Iglesias-Coma, Patricia Sánchez-Velázquez, Atypical double colorectal metastasis: spleen and uterus, Journal of Surgical Case Reports, Volume 2022, Issue 7, July 2022, rjab577, https://doi.org/10.1093/jscr/rjab577
Close - Share Icon Share
Abstract
Oligometastatic disease is a relatively new concept that refers to an intermediate stage between disseminated and localized cancer. Most frequent locations for colorectal metastasis are lung and liver. We present an a typical case of an 85-year-old woman who was diagnosed with a low-grade adenocarcinoma in left colon; she underwent a left laparoscopic hemicolectomy which resulted in a stage IIIb. After 24 months of follow-up, an increase of carcinoembryonic antigen (CEA) leads to the diagnosis of two metastatic lesions in two uncommon locations: spleen and myometrium. Stepwise surgical resection of both lesions was performed without complications. Spleen and uterus are organs that are rarely affected in colorectal cancer, the affection of both organs being even more infrequent. Despite the atypicality, surgical treatment is a valid strategy in this case of oligometastatic disease, which enables the disease-free survival of the patients.
INTRODUCTION
Colorectal cancer debuts as a metastatic disease at first presentation in up to 20% of cases and liver and lung metastases are the most frequent ones. Splenic metastases from colorectal origin are very rare (in the range 1.2–7.1%) [1] unlike other primary tumors such as melanoma, ovarian and breast cancer [2] and generally appear when the neoplastic disease is widely disseminated. On the other hand, involvement of the female genital tract as metastatic site of colorectal cancer is also somewhat unusual, ovaries being the most common location if presented. To the best of our knowledge there are no previous reports published about a synchronic uterus and splenic metastasis of colorectal cancer.
CASE REPORT
We present here the case of an 85-year-old woman with history of diabetes mellitus type 2 and erosive gastritis by Helicobacter pylori. In August 2018, she underwent a screening colonoscopy and was diagnosed with a low-grade adenocarcinoma in left colon and a tubulovillous adenoma in sigmoid colon. Staging computed tomography (CT) showed no disseminated disease; therefore, after presenting the case in the multidisciplinary tumor board a laparoscopic left hemicolectomy together with sigmoidectomy was performed. The pathologist report confirms that to be a low-grade adenocarcinoma pT3N1, with perineural and vascular invasion, and infiltration of three of 22 analyzed lymph nodes (stage IIIb).
In October 2018, the patient starts adjuvant chemotherapy with Capecitabin which was suspended in January 2018 due to toxicity. After 24 months of follow-up from surgery, CEA rose to 122 ng/ml and Ca 19.9 to 197 ng/ml. Therefore, a thoracoabdominal CT scan was performed to restage the disease and revealed an hypodense solid mass in the spleen measuring 40 × 55 × 40 mm, and a 41 × 42 × 43 mm uterine mass, both suggestive of metastatic lesions. The patient was once again discussed in the multidisciplinary tumor board, and it was decided to perform the surgery in two stages. Firstly, laparoscopic splenectomy was performed and 1 month later, the patient underwent a laparoscopic hysterectomy and double adnexectomy, as well as excision of right parametrial and ipsilateral pararectal nodules. Both surgeries presented an uneventful postoperative course.
The definitive pathological report revealed adenocarcinoma metastases with morphology and immunophenotype compatible with an intestinal origin, both in splenic parenchyma and in uterine myometrium (see Figs 1–8).
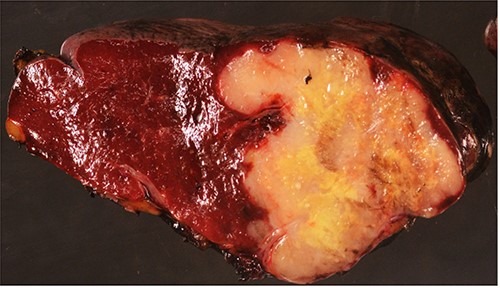
Surgical specimen: splenectomy; macroscopically, neoplastic retraction of the splenic capsule corresponding with a nodular, well-circumscribed, whitish neoplasm, 45 mm in diameter; parenchymatous resection margins are intact.
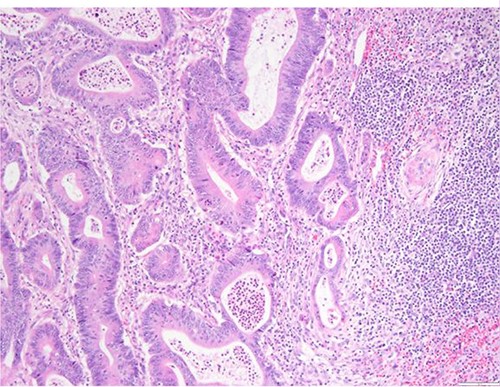
Surgical specimen: splenectomy; microscopy, HE; spleen parenchyma infiltrated by well-formed neoplastic glandular structures composed of cells with ‘pencillated’ and atypical nuclei, with nucleoli, apoptosis and mitotic figures, consistent with an intestinal origin (metastatic colorectal adenocarcinoma).
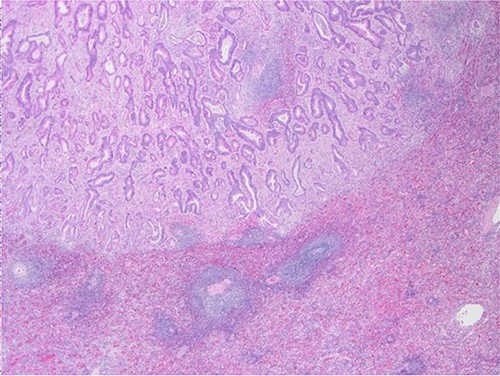
Surgical specimen: splenectomy; microscopy, HE; spleen parenchyma infiltrated by well-formed neoplastic glandular structures composed of cells with ‘pencillated’ and atypical nuclei, with nucleoli, apoptosis, and mitotic figures, consistent with an intestinal origin (metastatic colorectal adenocarcinoma).
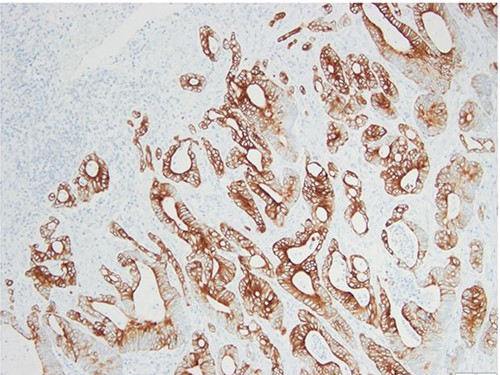
Surgical specimen: splenectomy; immunohistochemistry images: the neoplastic cells express CK20, consistent with an intestinal phenotype.
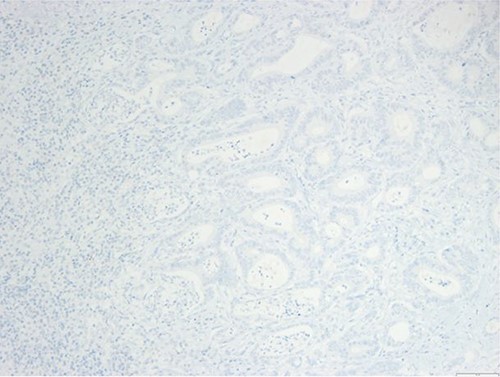
Surgical specimen: splenectomy; immunohistochemistry images: the neoplastic cells do not express CK7, consistent with an intestinal phenotype.
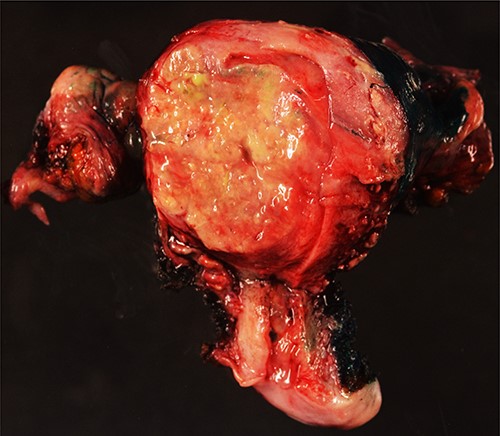
Surgical specimen: hysterectomy; macroscopically, an intramural nodular, yellowish, well-circumscribed neoplastic lesion, 45 mm in diameter, collapsing the endometrial cavity is seen.
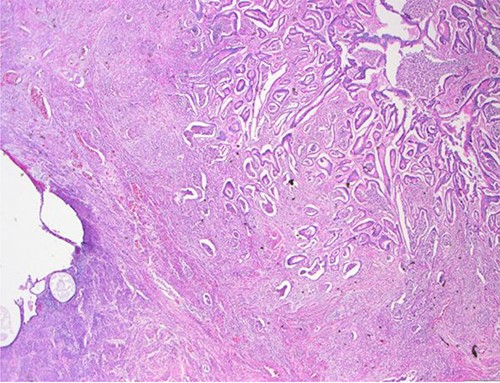
Surgical specimen: hysterectomy; microscopy, HE; endometrium and myometrium infiltrated by well-formed neoplastic glandular structures composed of cells with ‘pencillated’ and atypical nuclei, with nucleoli, apoptosis and mitotic figures, consistent with an intestinal origin (metastatic colorectal adenocarcinoma).
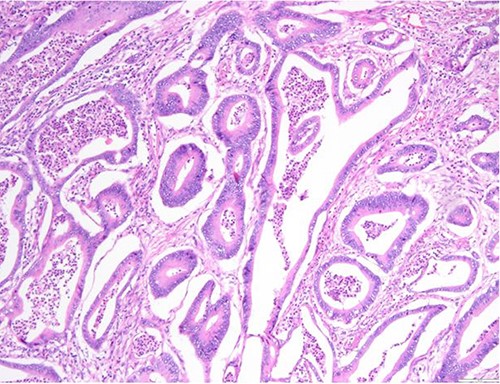
Surgical specimen: hysterectomy; microscopy, HE; endometrium and myometrium infiltrated by metastatic colorectal adenocarcinoma.
DISCUSSION
In contrast with other organs, spleen anatomy, histology and anatomy make it a hostile soil for neoplastic implantations. Anatomically, the sharp angle of the splenic artery with the celiac axis and rhythmic contraction by the sinusoidal splenic architecture are limiting factors for metastasis [3]. However, neoplastic cells are able to reach the parenchyma also via splenic vein, through a retrograde flow from inferior mesenteric vein or by its lymphatic vessels, explaining the capsular and subcapsular metastases. From an immunology point of view, its reticuloendothelial system appears to potently inhibit tumor cell proliferation [4], which makes the parenchymal seeding difficult. These are probably the reasons why the affectation of the spleen in colorectal metastatic disease normally appears when some other organs, such us liver, lung or axial skeleton are already affected [1, 5].
The affection of the female genital tract by metastatic disease finds its origin commonly in breast and gastrointestinal cancer. Ovaries are most often affected, while uterus is usually compromised only if ovaries have previously been involved. The rationale behind this is because uterine metastases are usually secondary to lymphatic spread from preceding ovarian affection, isolated uterine metastases without involvement of the ovaries are very rare and probably resulted not from lymphatic but from blood-stream spread [6]. In the last 10 years, there are few case reports describing isolated spleen metastasis from colorectal cancer; specifically, one interesting review from Abi Saad et al. [1] summarized all case-report articles, and conclude that the majority presented as metachronous metastasis in stage III left and sigmoid colorectal disease. Larger series of splenic metastases were mainly obtained from autopsy studies such as the one reported by Berge et al. [7], which found splenic metastasis in 21 of the 1019 colorectal cancer patients (2%), but all patients suffered from oligometastatic disease. Even though the diagnostic tools have significantly improved, the prevalence of splenic metastasis currently remains similar to the one described in classical autopsy studies and corresponds more frequently to melanoma, breast, lung, ovarian or gastric cancer and less common colorectal carcinomas.
This study is the first, to our knowledge, presenting a concomitant presentation of both uterus and splenic metastases.
In terms of management, oligometastasic disease might be an intermediate status between localized and diffused disease, and in these cases local control of the metastases might lead to improved outcomes [8]. Long-term survival can be obtained with surgical resection in colorectal metastases, particularly in patients with liver (and recently lung) affection. Significant survival can also be obtained with nonsurgical targeted approaches, as thermal ablation or stereotactic radiotherapy [9, 10].
CONCLUSIONS
Splenic and uterine metastases of colorectal origin are extremely rare, and even rarer is the combination of both in a patient. These patients are generally asymptomatic and diagnosed by elevation of tumor markers (mainly CEA) and by imaging tests (CT or MRI) during clinical follow-up. In these cases of oligometastatic disease, initial surgical treatment is an accepted option, as long as a radical surgery can be performed. Further studies are needed to discriminate phenotypes and predisposing factors to oligometastatic disease.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
Project PI20/00008, funded by Instituto de Salud Carlos III (ISCIII) and co-funded by the European Union.



