-
PDF
- Split View
-
Views
-
Cite
Cite
Shaikh B Iqbal, Abhinandan R Chittal, Shiavax J Rao, Pallavi Lakra, Deepty Bhansali, George Pyrgos, Spontaneous splenic rupture from indeterminate dendritic cell proliferation: a case report, Journal of Surgical Case Reports, Volume 2022, Issue 6, June 2022, rjac211, https://doi.org/10.1093/jscr/rjac211
Close - Share Icon Share
Abstract
Spontaneous splenic rupture is a rare and life-threatening phenomenon, usually associated with an underlying infectious, inflammatory, hematological, neoplastic or rheumatologic condition. Indeterminate cell tumor is a rare neoplastic dendritic cell disorder that is poorly understood but shares immunophenotypic markers for Langerhans cells without Birbeck granules. A 73-year-old man presented with upper abdominal pain after an unwitnessed fall. Computed tomography angiography showed splenomegaly and a large ruptured splenic subcapsular hematoma. Intraoperative findings from an emergency laparotomy revealed a large hemoperitoneum and a ruptured spleen. Microscopic sections identified numerous, mostly poorly formed, small nodules classified as a proliferation of indeterminate dendritic cell tumors.
INTRODUCTION
Splenic rupture is often precipitated by blunt trauma. Spontaneous splenic rupture (SSR) is a rare and life-threatening phenomenon, usually associated with an underlying condition [1]. Myelodysplastic syndromes are a heterogeneous group of clonal stem cells disorders within bone marrow characterized by ineffective hematopoiesis, peripheral cytopenias and dysplastic features with increased prevalence of transmission into acute myeloid leukemia [2]. Myeloid progenitor cells are precursors for dendritic cells. Indeterminate cell tumor (ICT) is a rare neoplastic dendritic cell disorder that is poorly understood but shares immunophenotypic markers for Langerhans cells without Birbeck granules. ICT is often reported in case reports with involvement of the skin [3]. We describe a unique case of indeterminate dendritic cell proliferation causing SSR in the setting of myelodysplastic syndrome.
CASE REPORT
A 73-year-old man presented after an unwitnessed fall with transient loss of consciousness for a few seconds. Medical history was remarkable for atrial fibrillation, basal cell carcinoma with recent resection, melanoma with positive axillary lymph nodes and myelodysplastic syndrome. He denied antecedent fever, chills, cough, chest pain, nausea, vomiting, diarrhea or constipation.
On presentation, he was afebrile, tachycardiac, mildly tachypneic, normotensive and requiring minimal oxygen supplementation. Physical exam revealed upper abdominal tenderness. Laboratory diagnostics showed normocytic anemia (hemoglobin 8.8 gm/dl; reference range: 12.5–16.5 gm/dl, mean corpuscular volume (MCV) 88.4 FL; reference range: 81–100 FL), leukocytosis (46.2 k/μl; reference range: 4.0–10.8 k/μl), lymphopenia (4.0 k/μl; reference range: 15.0–45.0 k/μl), thrombocytopenia (53 k/μL; reference range: 145–400 k/μL) and elevated lactic acid (5.2 mmol/l; reference range 0.7–2 mmol/l). Peripheral blood smear revealed occasional anisocytosis, polychromasia, ovalocytes, Dohle bodies and decreased platelet esterase.
Electrocardiogram showed sinus tachycardia (113 beats/minute). Computed tomography (CT) angiography of the chest, abdomen and pelvis was remarkable for splenomegaly and a large ruptured splenic subcapsular hematoma with moderate hemoperitoneum (Fig. 1). No potential vascular source of hemorrhage or masses were identified. He was initiated on a massive transfusion protocol with five units of packed red blood cells and two units of fresh frozen plasma. He received a dose of piperacillin–tazobactam and underwent emergent exploratory laparotomy with splenectomy. Intraoperative findings included a large hemoperitoneum with 3 l of dark blood around the spleen. The spleen was ruptured with a very large, separated capsule from the hematoma. Postoperatively, he developed a productive cough, with a left basilar opacity on X-ray, managed with antibiotics. He was eventually discharged with hematology/oncology outpatient follow-up.
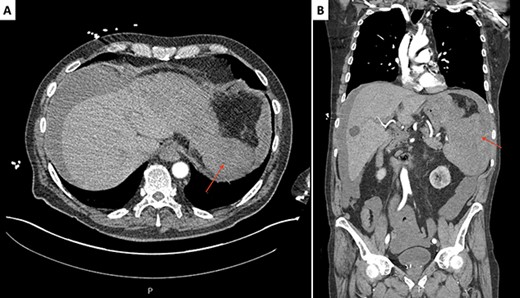
Initial CT scan of the abdomen and pelvis, axial (A) and coronal (B) slices, revealing a splenomegaly and a large ruptured supcapsular splenic hematoma (red arrows).
The surgical pathology report, which became later available, revealed no discrete tumor despite the enlargement of the spleen except for numerous, mostly poorly formed, small nodules within the red pulp. The nodules had normal histiocytes with more abundant atypical, but cytologically bland cells (Fig. 2) resembling Langerhans cells with irregular nuclear grooves and clefts. The cells expressed S100 (Fig. 3B), CD1a (Fig. 3B), CD4 and CD56. Immunostaining with Langerin, CD68 and melanoma markers were negative. The numerous small nodules were classified as a proliferation of indeterminate dendritic cell tumors.
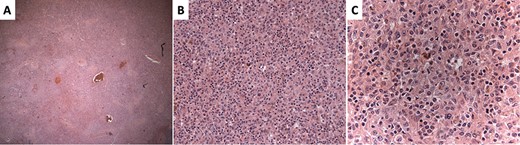
Hematoxylin and eosin–stained sections of the splenic red pulp. (A) ×4 magnification showing vague, nodular appearance. (B) ×10 magnification showing dendritic background with abundant eosinophilic cytoplasm. (C) ×20 magnification demonstrating atypical cells with cells resembling Langerhans cells showing irregular nuclear grooves and clefts.
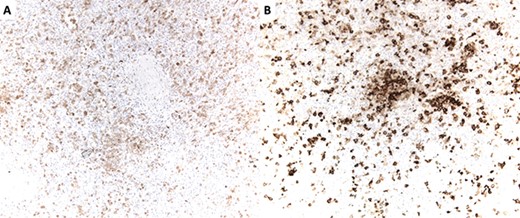
(A) S100 immunostain highlighting dendritic cells. S100 is also a marker of Schwann cells and melanocytes. (B) CD1a immunostain highlighting dendritic cells. CD1a is also an immunostain for Langerhans cells.
DISCUSSION
SSR is often associated with an underlying condition causing rapid splenic distention against a thin, tense capsule, with eventual rupture and hemorrhage. SSR accounts for 1% of all splenic ruptures, whereas 93% are associated with histopathological altered spleens. The altered spleens progressing to SSR have a mortality rate of 10–15% [1]. Predisposing conditions may be infectious, inflammatory, hematological, neoplastic or rheumatologic. ICT is a rare neoplastic disorder characterized by the presence of dendritic cells that immunophenotypically resemble Langerhans cells but lack Birbeck granules. Indeterminate cells are thought to be a precursor to Langerhans cells as both share a common precursor myeloid cell progenitor [3].
The usual presentation of SSR is acute abdomen and hypovolemic shock. Physical examination is usually notable for signs of peritonitis, referred left shoulder pain and dullness to percussion on both flanks except constant on the left despite the positional change. Common symptoms include nausea, vomiting and syncopal episodes [4]. Splenomegaly is found in myeloproliferative disorders; however, it is rarely associated with rupture [5].
Most presentations of ICT are lesions confined to the skin. Usually occurring in adults without predilection of sex and age, it can present asymptomatically as a single lesion or, less commonly, disseminated multifocal lesions occurring on the trunk, face, neck or extremities. ICT is rarely associated with other hematopoietic neoplasms [6]. Chen et al. [3] described the first case report of ICT in the spleen.
Orloff and Peskin’s criteria are used to establish the diagnosis of atraumatic idiopathic splenic rupture, which includes no history of trauma, no peri-splenic adhesions or scarring indicating previous trauma, no evidence of disease in organs other than the spleen that can cause rupture and normal spleen on gross and histological examination. After a full virological study, Crate and Payne added a fifth criterion: the absence of viral antibody titers. Focused assessment with sonography in trauma (FAST) is considered the best initial imaging method for unstable patients and can be easily performed in emergency departments. FAST can quickly and reliably reveal the presence of peritoneal fluid in the left upper quadrant [1].
Dendritic cells are antigen-presenting cells differentiated from colony-forming unit (CFU)-granulocytic/ monocytic cells into CFU–monocytic/dendritic cells and finally CFU–dendritic cells and CFU–monocytic cells. The CFU–dendritic cells differentiate into immature dendritic cells, Langerhans and interstitial dendritic cells. ICT requires immunophenotyping for diagnosis. Differentiating ICT from Langerhans histiocytosis is made by CD1a and S-100 protein expression and ultrastructurally lacking Birbeck granules. If ultrastructural identification cannot be performed, langerin immunostaining may be used as a surrogate marker, restricted to Langerhans cells [6].
Management of SSR is guided by the underlying etiology, hemodynamic stability, amount of blood products needed and the risk of post-splenectomy infections. In hemodynamically stable patients, non-surgical management can be achieved with splenic artery embolization [7]. In cases of idiopathic splenic rupture, splenectomy allows for final tissue diagnosis and intraoperative inspection of the abdomen for other potential causes for hemoperitoneum [4]. Our patient underwent an emergent splenectomy with the removal of hemoperitoneal fluid, allowing for definitive tissue diagnosis. The prognosis for ICT is dependent on its clinical stage. In a similar patient with ICT involvement in the spleen, splenectomy showed no disease progression [3].
CONFLICT OF INTEREST STATEMENT
None declared.



