-
PDF
- Split View
-
Views
-
Cite
Cite
Shahnawaz Haleem, Mohamed H Mahmoud, Gurvinder Singh Kainth, Rajesh Botchu, Md Faizul Hassan, 18F-FDG PET/CT scan standardised uptake value (SUV) score for diagnosis, staging and monitoring malignancy in spinal melanotic schwannoma, Journal of Surgical Case Reports, Volume 2022, Issue 12, December 2022, rjac524, https://doi.org/10.1093/jscr/rjac524
Close - Share Icon Share
Abstract
Melanotic schwannoma (MS) is a rare nerve sheath neuroectodermal neoplasm. We highlight the use of F18-fluorodeoxyglucose positive emission tomography/computed tomography (18F-FDG PET/CT) standardised uptake value (SUV) results in the diagnosis, staging and monitoring of spinal MS. A 58-year-old female patient had a 6-month history of left leg pain (S1) and no skin lesions. Magnetic resonance imaging reported a possible schwannoma with CT-guided biopsy, indicating a metastatic malignant melanoma. 18F-FDG PET/CT scan revealed only sacral destruction and an SUV score of 3.6. Histopathology results confirmed a malignant melanotic peripheral nerve sheath tumour (schwannoma). In MS, the 18F-FDG PET/CT scan SUV cut-off point can be used to distinguish between benign and malignant lesions, whereas (SUVmax) can predict the histologic response and therefore useful as a ‘screening test’. Our case highlights the increased uptake on PET/CT by melanocytic variant of neurogenic tumours and clinicians need to be aware of this.
INTRODUCTION
Melanotic schwannoma (MS) is a rare nerve sheath neuroectodermal neoplasm that accounts for < 1% of primary peripheral nerve sheath tumours and can also be present in disparate locations such as the acoustic nerve, cerebellum, orbit, soft tissue, choroids, heart, pancreas, trachea, bone, oral cavity and oesophageal wall [1]. Whereas conventional schwannomas are completely benign, MS is often considered benign but sometimes is potentially malignant, presented in a single form and known for secreting melanin from schwann cells [1, 2]. First reported in 1932, it usually occurs in the nerve roots and sympathetic nerve trunks [3]. It usually presents in the 30s with similar preponderance in both sexes [4, 5]. Whereas MS does not exhibit specific symptoms, its effect is related to its site, rate of growth and the consequences of nerve damage such as muscle weakness [3, 5]. Magnetic resonance imaging (MRI) reveals a high signal in T1 modality and low signal in T2 because of melanin content exhibiting paramagnetism properties. It has homogenous enhancement with contrast use [4–6]. F18-fluorodeoxyglucose positron emission tomography/computed axial tomography (18F-FDG PET/CT scan) is very accurate in diagnosing malignant MS revealing the degree, extent and ability to guide biopsy, detect occult lesions and is also useful in staging and monitoring the treatment, follow-up and prognosis [1, 6, 7]. It can therefore be used as a ‘screening test’ as it detects malignant tumours based on the glucose metabolism before histological/morphological changes but is a non-specific tumour detector as the inflammatory granuloma can show a high FDG uptake. Maximal standardised uptake value (SUVmax) therefore can predict the histologic response [8].
CASE PRESENTATION
In September 2018, a 58-year-old female patient presented to the referring unit with a 6-month history of left leg pain in the S1 nerve root distribution caused by a compressive lesion. The pain was present on standing, as night pain (7/10 on a Visual Analogue Score) and tingling with intermitted pain-free periods. Straight leg raise test gave 50° with no pain. She had an absent ankle jerk but no other neurological deficit. She had no bowel or bladder disturbance. Her pain was controlled with Naproxen. She had a past medical history of epilepsy, was a non-smoker with no previous malignancy and no history of weight loss. The MRI scan was reported as a possible Schwannoma and she was referred via the Bone Tumour Unit referral system.
The patient was discussed in the spinal MDT with a diagnosis of schwannoma and was reviewed 3 days later. She was noted to be allergic to Penicillin, her family history was negative for schwannoma and neurofibromatosis, and her neurological status was confirmed. Treatment strategies were discussed with the patient requesting some time to think things over.
At a further review (November 2018), a contrast MRI scan was requested at a follow-up in early November for 3 months post-index scan. At the follow-up, a left S1/S2 sacral tumour (most possibly schwannoma) with no change in the size was diagnosed. Repeat physical examination confirmed status quo on the neurological deficit. The decision was to review after 12 months as the patient remained stable.
Reviews in July 2019 (with an MRI scan in December 2019) and December 2020 confirmed no neurological deterioration but some increased back and leg pain when standing for some time. A repeat MRI scan confirmed an increase in the size of the lesion (Figs 1 and 2). A biopsy was arranged with a plan for a follow-up surgery. Results of the CT-guided biopsy indicated a diagnosis of metastatic malignant melanoma. Discussions with the patient did not localise any skin lesion responsible for the metastatic deposit, an F18-fluorodeoxyglucose positron emission tomography (18F-FDG PET/CT) scan and a conventional computed axial tomography (CT) scan was organised (April 2021), which revealed sacral destruction and no other deposits in the chest, abdomen and pelvis (Figs 3 and 4). Our patient had a reported SUV of 3.6, indicating a malignant lesion that was then confirmed operatively.
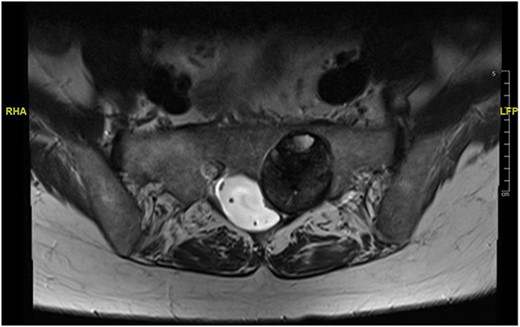
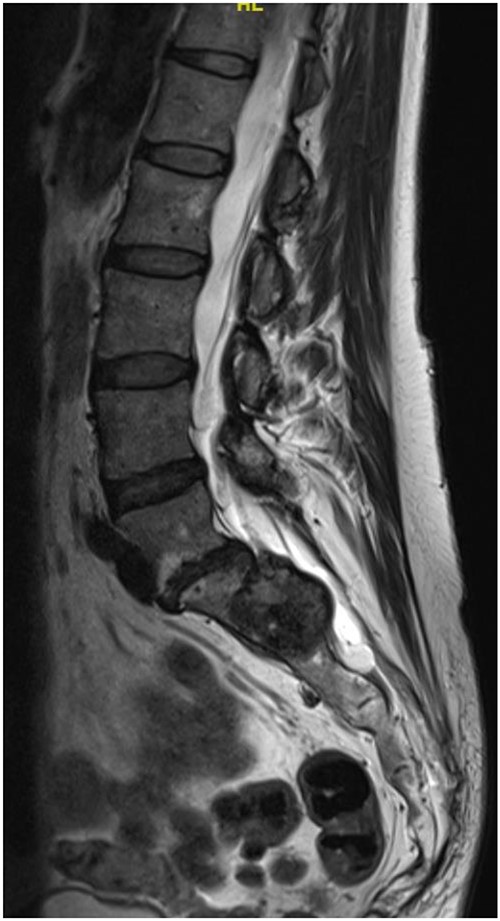
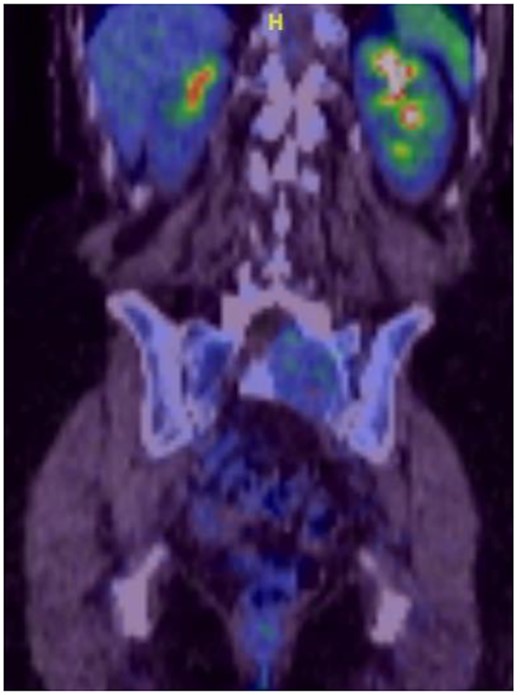
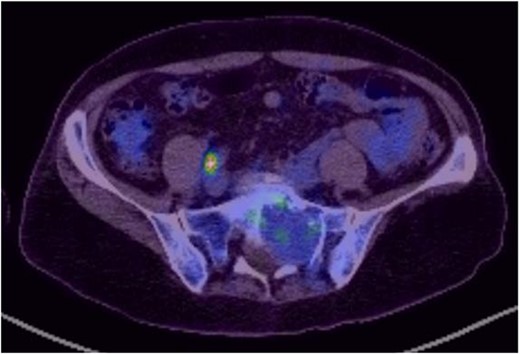
Intralesional debulking of the lesion was carried out with no complications in June 2021. Histopathology results confirmed a malignant melanotic peripheral nerve sheath tumour (schwannoma) (Fig. 5). She underwent a further procedure to attempt a complete excision of the lesion. Patient consent for publication was obtained.
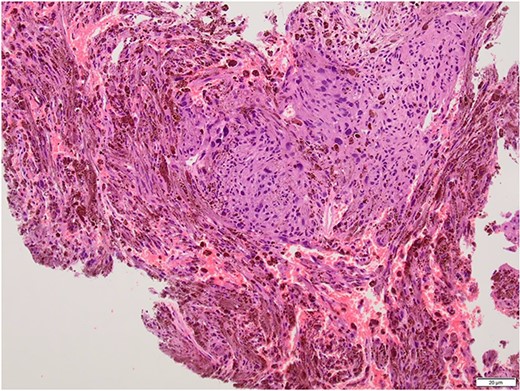
Histopathology slide with the tumour being composed of fascicles and pleomorphic cells obscured by the melanin pigment.
DISCUSSION
MS comprises <1% of primary peripheral sheath tumours. The most common sites are cervical and upper thoracic spinal nerves with 30–40 years being the most common age group of occurrences [1, 3, 9]. The MRI characteristics of high signal on T1WI and low signal on T2WI with homogenous enhancement with contrast are well known. Our patient was in an older age group during presentation and had no skin deposits. Whereas a diagnosis was made on biopsy, it is also possible to use the FDG uptake on PET/CT imaging to determine the benign or malignant nature of the tumour [1]. The standardised uptake value (SUV) is a simple way of determining activity in PET imaging, most commonly in FDG imaging, and can predict the histologic response [10, 11]. It is used to measure response of cancers to treatment and considered a semi-quantitative value as it is vulnerable to other sources of variabilities [10, 11]. Hamada et al. set the cut-off point to 3.0 for distinguishing benign and malignant lesions [12]. Whereas there is an overlap in the SUV cut-off point amongst various authors, there is also a wide variation between them [13, 14]. Ahmed et al. reported 0.33–3.7, whereas Aoki et al. reported 1.75 ± 0.84 [14, 15]. However, Aoki et al., in another paper, reported a wider range of 0.7–2.84 [13]. Our patient had a reported SUV of 3.6, indicating a malignant lesion that was then confirmed operatively.
In MS, the 18F-FDG PET/CT scan (SUV) cut-off point can be used to distinguish benign and malignant lesions. 18F-FDG PET/CT can be used as a ‘screening test’ as it detects malignant tumours based on the glucose metabolism before histological/morphological changes and can therefore be used in the diagnosis, staging and monitoring of spinal MS. Our case highlights the fact that the melanocytic variant of neurogenic tumours can show no skin lesions and an increased uptake on PET/CT of which clinicians need to be aware.
References
- magnetic resonance imaging
- fluorodeoxyglucose f18
- computed tomography
- biopsy
- cancer
- melanoma
- nerve sheath tumors
- neurilemmoma
- neuroectodermal tumors
- acoustic neuroma
- diagnosis
- skin lesion
- lower limb pain
- computed tomography/positron emission tomography imaging
- nerve sheath
- histopathology tests
- neurogenic tumors



