-
PDF
- Split View
-
Views
-
Cite
Cite
Wajih Sahnoun, Marouene Chakroun, Selim Zaghbib, Ahmed Saadi, Ahlem Blel, Soumaya Rammeh, Haroun Ayed, Mohamed Chebil, Management and prognosis of liposarcomas of the spermatic cord: an experience with six cases, Journal of Surgical Case Reports, Volume 2022, Issue 1, January 2022, rjab621, https://doi.org/10.1093/jscr/rjab621
Close - Share Icon Share
Abstract
Liposarcoma of the spermatic cord (LSC) is a rare tumor with no consensus on therapeutic management. This study reports six new cases of LSC. The patients’ age ranged from 56 to 80 years. All patients presented with a scrotal mass, and it was ultrasound that oriented the diagnosis. The initial treatment consisted of an inguinal orchiectomy. Anatomopathological study coupled with immunohistochemistry using the anti-MDM2 antibody confirmed that the tumors were well-differentiated LSC in four cases and dedifferentiated LSC in the other two cases. Adjuvant radiotherapy was performed in two patients. No recurrence was noted in these two patients at 14 and 34 months of follow-up. The only recurrence we had was local and occurred at 44 months of follow-up in a patient who had a dedifferentiated form ofLSC.
INTRODUCTION
Liposarcomas of the spermatic cord (LSC) are a rare entity, and there are few reported cases in the literature. Given the high frequency of locoregional recurrence, treatment is based on complete surgical removal of the tumor. Adjuvant radiotherapy can be proposed with interesting results. Six cases of LSC diagnosed between 2012 and 2020 are reported.
This study aims to describe the diagnostic, therapeutic management and prognosis ofLSC.
CASE SERIES
Between 2012 and 2020, six cases of LSC were diagnosed using an anatomopathological examination coupled, in four cases among 6, with an immunohistochemical study using the anti-MDM2 antibody. From the patients’ medical records, the age of subjects, the size of the tumor, its side, the histological type, the quality of the surgical margins, the number and nature of the surgical interventions, the local and metastatic recurrences and their delays as well as the complementary treatments were noted.
Complete data were obtained in five out of six patients; one patient was lost to follow-up after 1 year of postoperative monitoring. The motive for consultation in all cases was a scrotal swelling evolving progressively withoutpain.
Testicular ultrasonography suggested the diagnosis in all cases, showing a hypoechoic extra testicular scrotal mass highly vascularized at Doppler (Fig. 1).
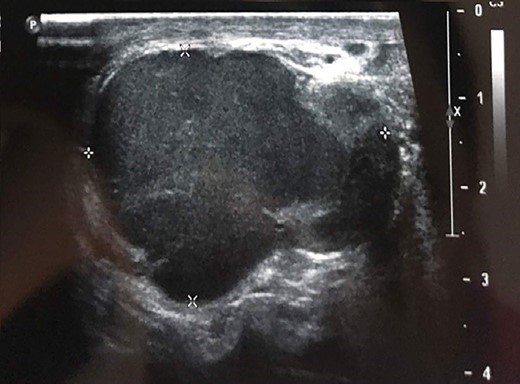
Testicular ultrasound showing a hypoechoic left extra testicular scrotal mass that is highly vascularized at Doppler.
In four cases, the tumor was located on the left side. The major tumor axis ranged between 4 and 10 cm. Testicular tumor markers (AFP, HCG and LDH) were negative in all patients.
The first treatment was a radical inguinal orchiectomy with tumor removal in all cases (Fig. 2).
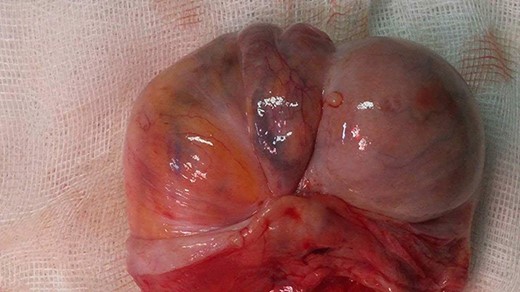
Left radical orchiectomy specimen containing a spermatic cord tumor.
An anatomopathological examination of the surgical specimens coupled, in four out of six cases, with an immunohistochemical study using the anti-MDM2 antibody concluded to a well-differentiated LSC in four cases and a dedifferentiated LSC in the two other cases (Fig. 3).
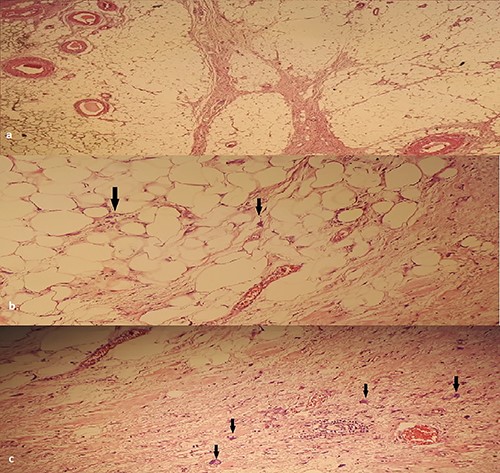
(a) HEX4: Lobules of adipocyte cells separated by fibrous septa. (b) HEX400: large nuclei, (c) HEX400: atypical cells: enlarged nuclei with irregular contours.
The surgical margin on the spermatic cord was healthy in five cases. The 6th case had a tumoral margin and had a complementary surgical resection with an anatomopathological examination that did not reveal any tumoral residue. In five cases, no recurrence was noted (follow-up from 11 to 72 months). The only recurrence that occurred was local and occurred at 44 months of follow-up in a patient who had a well-differentiated LSC with a dedifferentiated contingent. Table 1 summarizes the surgical and pathology data for our patients.
| Patient . | First treatment . | Tumor size (cm) . | Anatomopathological examination . | Quality of the excision (margins) . | Complementary treatment . | Recurrence . | Recurrence treatment . |
|---|---|---|---|---|---|---|---|
| 1 | Left inguinal orchiectomy | 9.5 | Dedifferentiated liposarcoma | Complete | None | None | |
| 2 | Right inguinal orchiectomy | 7 | Well-differentiated liposarcoma with dedifferentiated contingent | Complete | Local recurrence | Surgical excision: Dedifferentiated liposarcoma (6.5 cm) Healthy margins | |
| 3 | Left inguinal orchiectomy | 5 | Well-differentiated liposarcoma | Incomplete (Tumoral margin on spermatic cord) | • Complementary Surgical excision (Non-tumoral margins on specimen examination) | None | |
| • Radiotherapy | |||||||
| 4 | Left inguinal orchiectomy | 4 | Well-differentiated liposarcoma | Complete | None | ||
| 5 | Right inguinal orchiectomy | 8 | Well-differentiated liposarcoma sclerosant subtype | Complete | Radiotherapy | None | |
| 6 | Left inguinal orchiectomy | 10 | Well-differentiated liposarcoma | Complete | None |
| Patient . | First treatment . | Tumor size (cm) . | Anatomopathological examination . | Quality of the excision (margins) . | Complementary treatment . | Recurrence . | Recurrence treatment . |
|---|---|---|---|---|---|---|---|
| 1 | Left inguinal orchiectomy | 9.5 | Dedifferentiated liposarcoma | Complete | None | None | |
| 2 | Right inguinal orchiectomy | 7 | Well-differentiated liposarcoma with dedifferentiated contingent | Complete | Local recurrence | Surgical excision: Dedifferentiated liposarcoma (6.5 cm) Healthy margins | |
| 3 | Left inguinal orchiectomy | 5 | Well-differentiated liposarcoma | Incomplete (Tumoral margin on spermatic cord) | • Complementary Surgical excision (Non-tumoral margins on specimen examination) | None | |
| • Radiotherapy | |||||||
| 4 | Left inguinal orchiectomy | 4 | Well-differentiated liposarcoma | Complete | None | ||
| 5 | Right inguinal orchiectomy | 8 | Well-differentiated liposarcoma sclerosant subtype | Complete | Radiotherapy | None | |
| 6 | Left inguinal orchiectomy | 10 | Well-differentiated liposarcoma | Complete | None |
| Patient . | First treatment . | Tumor size (cm) . | Anatomopathological examination . | Quality of the excision (margins) . | Complementary treatment . | Recurrence . | Recurrence treatment . |
|---|---|---|---|---|---|---|---|
| 1 | Left inguinal orchiectomy | 9.5 | Dedifferentiated liposarcoma | Complete | None | None | |
| 2 | Right inguinal orchiectomy | 7 | Well-differentiated liposarcoma with dedifferentiated contingent | Complete | Local recurrence | Surgical excision: Dedifferentiated liposarcoma (6.5 cm) Healthy margins | |
| 3 | Left inguinal orchiectomy | 5 | Well-differentiated liposarcoma | Incomplete (Tumoral margin on spermatic cord) | • Complementary Surgical excision (Non-tumoral margins on specimen examination) | None | |
| • Radiotherapy | |||||||
| 4 | Left inguinal orchiectomy | 4 | Well-differentiated liposarcoma | Complete | None | ||
| 5 | Right inguinal orchiectomy | 8 | Well-differentiated liposarcoma sclerosant subtype | Complete | Radiotherapy | None | |
| 6 | Left inguinal orchiectomy | 10 | Well-differentiated liposarcoma | Complete | None |
| Patient . | First treatment . | Tumor size (cm) . | Anatomopathological examination . | Quality of the excision (margins) . | Complementary treatment . | Recurrence . | Recurrence treatment . |
|---|---|---|---|---|---|---|---|
| 1 | Left inguinal orchiectomy | 9.5 | Dedifferentiated liposarcoma | Complete | None | None | |
| 2 | Right inguinal orchiectomy | 7 | Well-differentiated liposarcoma with dedifferentiated contingent | Complete | Local recurrence | Surgical excision: Dedifferentiated liposarcoma (6.5 cm) Healthy margins | |
| 3 | Left inguinal orchiectomy | 5 | Well-differentiated liposarcoma | Incomplete (Tumoral margin on spermatic cord) | • Complementary Surgical excision (Non-tumoral margins on specimen examination) | None | |
| • Radiotherapy | |||||||
| 4 | Left inguinal orchiectomy | 4 | Well-differentiated liposarcoma | Complete | None | ||
| 5 | Right inguinal orchiectomy | 8 | Well-differentiated liposarcoma sclerosant subtype | Complete | Radiotherapy | None | |
| 6 | Left inguinal orchiectomy | 10 | Well-differentiated liposarcoma | Complete | None |
Adjuvant radiotherapy was performed in two of our patients, one had a well-differentiated LSC of 8 cm and the other a well-differentiated LSC of 5 cm long axis but with positive margins at the time of surgical resection. No local recurrence was noted at 34 months and 14 months of follow-up in these two patients.
None of our patients had retroperitoneal lymph node dissection or chemotherapy treatment.
DISCUSSION
LSC is a rare entity that has been poorly documented in the literature. The largest and only prospective study is reported by Coleman et al. with 47 cases from 1982 to 2001.
Among all sarcomas of the testicular region, LSCs are by far the most frequent, as our small series shows. Clinically, the differential diagnosis with a lipoma can be difficult. Rapid evolution, large size and the presence of symptoms are criteria in favor of malignancy. Contrary to the literature data showing that the LSC occurs more frequently on the right side, four of six patients had developed the tumor on the left side in our study [1].
On ultrasound, LSC appears as hyperechoic and heterogeneous solid formations. On computed tomography (CT) scan, it appears as a fattymass.
The classification of LSCs is based on their histological grade and the extension assessment. The World Health Organization (WHO) classifies soft tissue tumors into five groups of LSC with increasing aggressiveness: (i) well-differentiated LSC, which includes adipocytic, inflammatory and sclerosing subtypes; (ii) dedifferentiated LSC; (iii) myxoid; (iv) round cell and (v) pleomorphic [2, 3] The new WHO classification merges myxoid LSC and round cell LSC, because the latter is simply a high-grade variant of the former, and it is common to see a transition from one to the other in the same tumor [1].
Well-differentiated or lipoma-like LSC is characterized by a proliferation of mature adipocytes of variable size and shape, associated with spindle atypical stromal cells with hyperchromatic nuclei predominating in fibrous septa. Differential diagnosis with a lipoma is particularly difficult if there are very few fibrous areas. In this situation, immunohistochemistry for overexpression of the MDM2 and CDK4 genes is of interest although it is neither sensitive nor 100% specific [4]. The aggressiveness of LSC increases with the extent of the areas of dedifferentiation. Dedifferentiation can be found immediately or can occur during a recurrence of a well-differentiated LSC, underscoring the importance of complete exeresis [5].
The curative treatment of LSC is essentially based on a broad excision including an orchiectomy with healthy surgical margins. In the study by Coleman et al. [6], healthy surgical margins are associated, in a multivariate study, with a decrease in the recurrence rate and an increase in survival. It is, therefore, preferable to discuss orchiectomy with the patient before surgery. Retroperitoneal lymph node dissection is proposed when lymph node involvement is evident and in the case of embryonic rhabdomyosarcoma due to the higher risk of lymph node metastasis.
LSCs are characterized by their fairly frequent local recurrence (30–50% of cases), which can occur after a period of up to 5 years. This implies surveillance over several years. This local recurrence rate is high regardless of tumor size and histological type. Local adjuvant treatment with radiotherapy has not formally demonstrated its efficacy concerning local recurrence and overall survival as was shown in the study by Coleman et al. Nevertheless, in other series, the beneficial effect of adjuvant radiotherapy on the reduction of local recurrence has been demonstrated.
Unlike rhabdomyosarcoma, where the role of chemotherapy is proven, the effect of systemic treatment remains controversial.
Because of the relatively high rate of local recurrence, regular postoperative monitoring is recommended. It is based on a clinical examination and a thoraco-abdominopelvic CT scan every 6 months for 2 years, then once ayear.
CONCLUSION
In the presence of any palpable solid mass at the para testicular or inguinal canal level, the diagnosis of liposarcoma must be suspected. Wide surgical excision is essential. Adjuvant treatments have not proven to be effective. A regular postoperative follow-up in seeking recurrences is mandatory.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this case report and accompanying images.
FUNDING STATEMENT
The authors declare that they did not receive any funding regarding this article.



