-
PDF
- Split View
-
Views
-
Cite
Cite
Brian O’Sullivan, Thomas Burton, Ralph Van Dalen, Fraser Welsh, Archana Pandita, Jesse Fischer, Beware the pancreatic incidentaloma in colorectal tumours: pancreatic adenocarcinoma with metastases to the colon and rectum, Journal of Surgical Case Reports, Volume 2022, Issue 1, January 2022, rjab629, https://doi.org/10.1093/jscr/rjab629
Close - Share Icon Share
Abstract
Colorectal cancer (CRC) is the third most diagnosed malignancy in the Western world. Routine staging of CRC often identifies incidental lesions on cross-sectional imaging. Appropriate treatment is dependent on a correct histological diagnosis. Pancreatic Ductal Adenocarcinoma (PDAC) is a rarer and often devastating diagnosis for which the treatment pathway differs significantly to CRC. We report two rare cases: the first recorded case of PDAC with synchronous rectal metastasis and a case of an acute presentation with large bowel obstruction from synchronous colonic metastasis. Both cases presented a significant diagnostic challenge. The management of both cases would have been altered had the histological diagnosis been known prior to surgery. Clinicians treating CRC should be wary of incidental lesions on staging investigations as they rarely represent an occult extra-intestinal primary malignancy. Immunohistochemistry plays an important role in ascertaining the origin of gastrointestinal malignancy.
INTRODUCTION
Colorectal tumours are common and usually due to neoplastic transformation at various stages of the adenoma-carcinoma pathway [1]. Colorectal cancer (CRC) is the third most diagnosed malignancy in the Western world, with an estimated incidence of 38.7 per 100 000 persons per year [2]. Rectal adenocarcinoma accounts for almost one-third of all CRC [2]. Standard of care for rectal cancer includes neoadjuvant radiotherapy for locally advanced tumours (clinically T3 or above or lymph node positive). Histological confirmation of adenocarcinoma is recommended prior to treatment of CRC, but is particularly important when considering neoadjuvant therapy for rectal cancer [3]. Pancreatic ductal adenocarcinoma (PDAC) is a rarer but often devastating diagnosis; <20% of patients have surgically resectable disease at presentation and overall 5-year survival is ~5% [4]. Metastases to liver and lung are common and account for 90% of PDAC-associated deaths [5]. Metastatic disease to the gastrointestinal tract from PDAC is rare [6]. We report two cases of PDAC with synchronous colorectal metastases, each presenting a significant diagnostic challenge.
CASE SERIES
Case A
An 82-year-old man with no significant medical history presented with progressive change in bowel habit and abdominal pain for 2 months. Colonoscopy 1 year prior had only revealed diverticulosis but on rectal examination at re-presentation a firm mass was palpable within the rectum. Colonoscopy was repeated and revealed an ulcerated non-obstructing low-rectal mass 3 cm in size (Fig. 1). Biopsies reported adenomatous change with at least high-grade dysplasia and a focus suspicious for invasive adenocarcinoma.
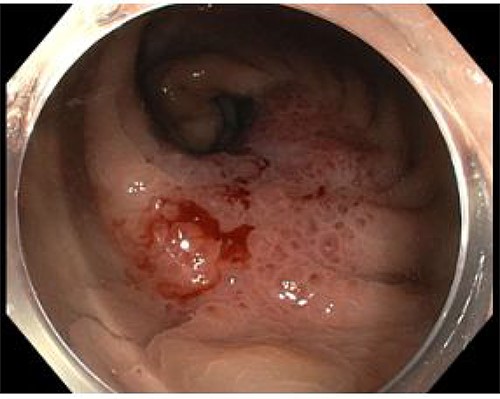
Case A. Endoscopic image at time of initial diagnosis with rectal tumour, prior to neoadjuvant chemoradiation.
Routine staging for rectal cancer was performed. Magnetic resonance imaging (MRI) of the pelvis revealed an anterior rectal mass 7–8 cm from the anal verge with invasion into the seminal vesicles and at least three lymph nodes suspicious for metastases (cT4aN2). Computed tomography (CT) chest, abdomen and pelvis revealed enlarged nodes along the inferior mesenteric artery chain, but no definite metastatic disease was reported (Fig. 2). A 1.4 cm low attenuation hypo-enhancing mass in the distal pancreas occluding the splenic vein was reported as an incidental finding. Serum carcinoembryonic antigen (CEA) was 1.71ug/L and serum carbohydrate antigen 19-9 (Ca 19-9) was mildly elevated at 78u/ml.
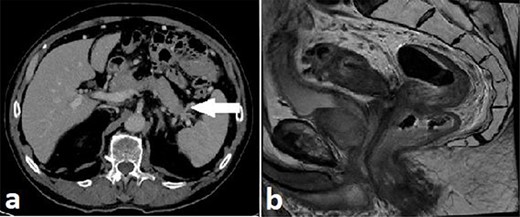
Case A. Axial portal venous phase CT scan demonstrating hypo-enhancing distal pancreatic lesion encasing splenic vessels (a) and sagittal MRI pelvis demonstrating anterior low rectal tumour (b), staged as a cT4aN2Mx rectal adenocarcinoma.
Multidisciplinary team (MDT) discussion involving a pancreatic surgeon deemed a primary rectal adenocarcinoma with either incidental primary malignant or metastatic lesion in the distal pancreas as the likely diagnoses. Treatment recommended was neoadjuvant long-course chemoradiotherapy for the rectal tumour followed by rectal resection and distal pancreatectomy. Following neoadjuvant therapy restaging showed a slight reduction in size of pancreatic tumour from 14 to 12.6 mm and was again reported as consistent with a metastasis from a primary rectal malignancy. A laparoscopic ultra-low Hartmann’s procedure (gastrointestinal continuity not restored due to functional status preoperatively) combined with distal pancreatectomy and splenectomy was performed.
Histopathology revealed a well to moderately differentiated, 18 mm PDAC in the pancreatic tail with lymphovascular and perineural invasion. The rectal specimen revealed a 32 mm mass in the anterior rectum and microscopically demonstrated grossly intact colorectal mucosa with extensive infiltration by adenocarcinoma from the sub-serosal aspect. The tumour in the submucosa and underlying structures of rectum showed immunoreactivity for CK7 while CK20 and CDX2 were negative; however, the overlying rectal mucosa showed reactivity to CK20 and CDX2 but was negative for CK7 (Fig. 3).
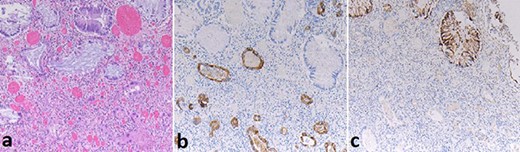
Case A. Specimen x10 magnification showing rectal metastasis from pancreatic ductal adenocarcinoma (a) with positive submucosal CK7+ immunostaining (b) and mucosal CK20+ immunostaining (c) but negative submucosal CK20 immunostaining(c).
Case B
A 71-year-old man with a background of cardiac disease and obstructive sleep apnoea presented to the emergency department with symptoms of large bowel obstruction. Urgent CT abdomen and pelvis revealed short segment hepatic flexure thickening and stranding with evidence of obstruction at this point. An additional soft tissue mass in the tail of the pancreas encasing the splenic vasculature was reported as suspicious for metastatic disease from a primary colonic malignancy or a synchronous primary pancreatic malignancy (Fig. 4). Serum CEA was 5.32ug/L and serum Ca 19-9 was significantly elevated at 1180u/ml.
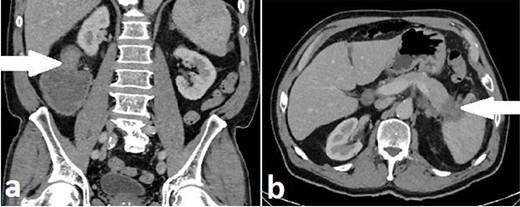
Case B. Portal venous phase CT scan of the abdomen. Coronal view of short segment hepatic flexure thickening (arrow) with proximal large bowel obstruction (a) and axial view of soft tissue mass (arrow) at tail of pancreas (b).
Due to the presence of large bowel obstruction, the patient proceeded to an urgent laparotomy. Operative findings were of a hard sclerotic mass within the pancreatic tail involving the splenic hilum. A further cicatrizing tumour was identified at the hepatic flexure with obstruction of the ascending colon. A right hemicolectomy and distal pancreatectomy with en-bloc splenectomy was performed.
Histopathology of the pancreatic resection revealed a 45 mm moderately differentiated PDAC with lymphovascular and perineural invasion. The colonic specimen revealed infiltration of metastatic PDAC from the serosal aspect into the muscularis and submucosa with focal areas infiltrating into mucosa. One of 18 mesocolic lymph nodes resected contained PDAC. Immunostaining of both tumours was positive for CK7 and negative for CK20.
DISCUSSION
We report two cases of PDAC with synchronous colorectal metastases from the same institution within a 4-month period. In both instances a presumptive diagnosis of CRC with either distant metastasis to the pancreas or incidental pancreatic tumour was made, and treatment proceeded according to that recommended for CRC. If the true diagnosis was known the management of both cases could have differed significantly.
CRC is common and in a patient with lower gastrointestinal symptoms, imaging and/or histology suggestive of a primary CRC this is likely to be the presumed diagnosis, even in an MDT setting with numerous CRC specialists and a pancreatic surgeon. Pancreatic lesions are metastases in only 0.5–3% of cases [7] and pancreatic metastases from a colorectal primary are rare with only isolated case reports in the literature [8–10]. When distant CRC metastases are present, modern management tends to support multi-visceral resection if complete cytoreduction is possible [11], hence aggressive surgical treatment was pursued in both cases. The worldwide incidence of PDAC has more than doubled in the past 20 years [4] and will likely continue to grow with our ageing population. PDAC carries a poor prognosis and surgery is the only potentially curative treatment, but distant metastasis is a contra-indication to attempting curative resection [4, 5].
Metastasis of PDAC to the colon and rectum is extremely rare but may become more common with increasing incidence of PDAC. We describe the first reported case of PDAC with synchronous rectal metastasis, which was also occult. There is a single previous case report of PDAC metastasizing to the rectum [12], but this was a metachronous event in a patient with a history of pancreatic cancer resection and elevated pancreatic tumour markers. While the pancreatic lesion in case A was suspicious for malignancy, the possibility of a rectal metastasis from a PDAC was never strongly considered. The patient subsequently received routine treatment for locally advanced rectal cancer in our institution at the time; this treatment would not have been recommended had the diagnosis been known preoperatively and indeed non-operative management with palliative chemotherapy would usually be recommended for stage 4 PDAC. There have been six previous case reports of synchronous colonic metastases from PDAC [13–18], three reports of metachronous metastases from PDAC [12, 19, 20] and one report of metachronous colonic metastases from pancreatic acinar cell cancer (PACC) [21]. Pancreatic metastases were either confirmed or suspected preoperatively in three of those with synchronous disease [13, 16, 17] and two of those with metachronous disease [20, 21].
The rarity of colorectal metastasis from PDAC and lack of discerning imaging characteristics means radiological reporting is unlikely to suggest a pancreatic primary as the most likely diagnosis. Colonoscopy and biopsy should be performed when possible; the endoscopic appearance may not appear typical of a mucosal lesion such as CRC (Fig. 4). In case B, the presence of large bowel obstruction precluded colonoscopy; synchronous resection was performed due to concern post-operative recovery after right hemicolectomy could significantly delay elective pancreatic resection.
Along with the morphological histopathological features, immunohistochemistry strongly supported the diagnosis of PDAC metastasis following resection in both cases. Immunostaining was not performed on the diagnostic biopsy in Case A which showed tubular adenoma with a focus of high grade dysplasia; if only superficial rectal mucosal biopsies were obtained it may still have stained as for a CRC primary with immunohistochemistry. Histological diagnosis was not available preoperatively for Case B. Cytokeratin’s are intermediate filament proteins found in the intracytoplasmic skeleton of epithelial cells and are useful markers for epithelial malignant proliferations [22], CK7 immunostain is positive in upper gastrointestinal glandular epithelium and CK20 positive in lower gastrointestinal glandular epithelium. The colorectal specimens from both Case A and Case B show immunoreactivity for CK7 but not CK20 in the submucosal adenocarcinoma. In these clinical settings this finding is highly suggestive of metastatic PDAC and corresponds with the reported literature [12–20]. Mucosal dysplasia seen at diagnostic biopsy was not present in the resection specimen, and was presumed treated by chemoradiotherapy. Immunohistochemistry played an important vital role in determining the tumour tissue origin in both cases.
Case A showed mildly elevated serum CA19-9 and normal serum CEA levels. Two other case reports showed this trend, one being a synchronous metastasis [13] and the other a metachronous metastasis [19]. Case B showed elevated serum CA19-9 and minimally elevated serum CEA levels. Other cases had significant rises in both tumour markers [12, 14–16, 20], and while a high CA19-9 will alert the clinicians to the possibility of a PDAC, a low/normal CA19-9 does not exclude it. CEA may or may not be elevated in either diagnosis.
In conclusion, we report two rare cases of synchronous colorectal metastasis from PDAC in which the diagnosis was not known until after combined colorectal and pancreatic resection. The management of both cases, but case A (rectal metastasis) in particular, would have been significantly altered had the histological diagnosis been known prior to treatment. Immunohistochemistry is an important tool in determining the primary origin of a gastrointestinal malignancy and should be performed on pre-treatment biopsy where there is clinical possibility of an alternative primary site; this is particularly critical for rectal tumours where neoadjuvant therapy may be recommended.
ACKNOWLEDGEMENTS
Both patients have given their informed consent for this case report to be written and submitted for publication.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- immunohistochemistry
- cancer
- colorectal cancer
- gastrointestinal cancer
- colorectal neoplasms
- intestines
- neoplasm metastasis
- surgical procedures, operative
- western world
- colon
- diagnosis
- diagnostic imaging
- pancreas
- rectum
- pancreatic adenocarcinoma
- large intestinal obstruction
- pancreatic ductal adenocarcinoma
- metastasis to the colon
- metastasis to the rectum



