-
PDF
- Split View
-
Views
-
Cite
Cite
Sandrine Darigny, Parla Astarci, Maxime Elens, A rare case of spontaneous superficial femoral artery pseudoaneurysm in a young patient: case report and review of literature, Journal of Surgical Case Reports, Volume 2021, Issue 8, August 2021, rjab327, https://doi.org/10.1093/jscr/rjab327
Close - Share Icon Share
Abstract
Spontaneous femoral artery pseudoaneurysm (PSA) is a rare disease and there are few reported cases. We report a case of a 17-year-old male with increasing left leg pain associated with swelling at the site of the pain. We observed a voluminous pulsatile mass. He had no history of trauma or surgery. Imaging confirmed a large PSA of the proximal portion of the left superficial femoral artery (SFA). The PSA was treated by resection of the aneurysm, reconstruction with inter-positional saphenous vein graft. Three months later; he came back to the emergency room for a pulsatile mass. The scan showed a PSA of his left SFA and a hematoma with active bleeding. It was treated surgically by resection of the aneurysm and reconstruction with graft.
INTRODUCTION
A pseudoaneurysm (PSA) is a focal widening of the vascular lumen due to partial or complete rupture of the arterial wall and contained bleeding. Leaking blood is contained either by the surrounding tissue or by the intact middle or adventitious tunica layers [1].
Spontaneous PSA of the superficial femoral artery (SFA) in young people is rare. The etiology of PSA includes infection, traumatic causes, Behçet's disease, Ehlers-Danlos syndrome (type IV) and other connective tissue disorders.
Often, PSAs of the femoral artery have been described following vascular interventions. It has become a common complication since the generalization of access through the femoral artery for endovascular procedures [2].
Some of the false aneurysms may close spontaneously, but rupture is a major problem, followed by distal embolization, thrombosis and compression of adjacent structures. Fortunately, spontaneous rupture of the artery without trauma is rare.
CASE REPORT
A 17-year-old man presented to the emergency department with increasing leg pain associated with swelling at the site of the pain. He had been experiencing pain of moderate intensity in the mid third of his left thigh for ~7 days. Symptoms gradually worsened over the last 2 days.
He was a factor V leiden carrier and had a history of cerebral venous thrombosis. He was being treated with therapeutic anticoagulation. He had no other comorbidities. He had no history of smoking had no history of trauma or surgery.
On physical examination, he was tachycardic (103 bpm) with a blood pressure of 130/70 mmHg. We demonstrated a large pulsatile mass of the left upper leg (Fig. 1). His pulses were palpable and there was no evidence of distal embolization.

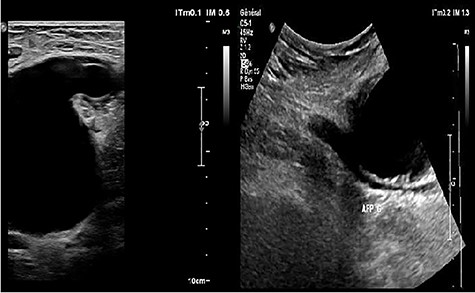
Ultrasonography (US) of the left thigh showed an aneurysmal dilatation of the SFA measuring 67 × 50 × 80 mm (Fig. 2) on the anterior aspect of the proximal third of the thigh. Computer tomography angiogram (CTA) confirmed a 64 × 80 × 87-mm PSA developed on the anterior side of the proximal left SFA (Fig. 3). The other arterial axes were healthy and normal. Supplementary imaging examinations did not identify any additional aneurysms.
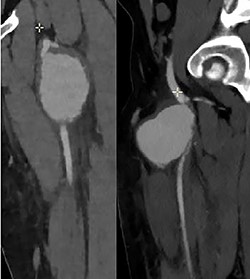
Computer tomography angiogram showed a PSA of the proximal SFA.
After a multidisciplinary discussion, we decided to wait until the effect of anticoagulation ceased before operating on the patient.
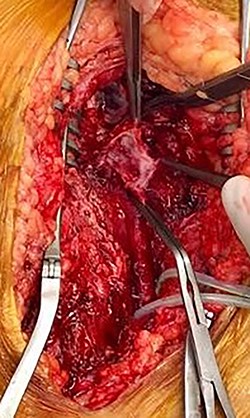
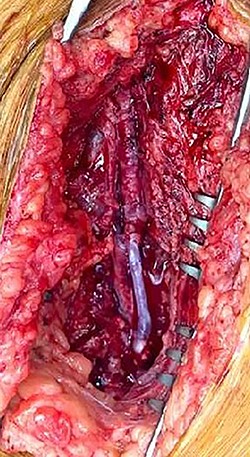
During operative intervention, a hematoma was observed involving muscle tissues in the anteromedial region of the thigh. There were no obvious signs of active infection. The proximal control obtained, the aneurysm incised and the distal control obtained. The aneurysm resected and samples collected for anatomopathological and microbiological analyses (Fig. 4). Reconstruction was performed by interposition of the ipsilateral great saphenous vein in reverse, with end-to-end anastomosis taking into consideration the diameter of the femoral artery (Fig. 5). Intraoperative tissue cultures were negative and the anatomopathological analysis showed a histopathological aspect corresponding to a PSA of the femoral artery, with the absence of the arterial wall and without specific character.
At 2-month follow-up, the patient had palpable distal pulses and was free from pain or other problems. The control echodoppler shows triphasic flow within the left common and superficial femoral arteries.
At 3-month follow-up, he presented to emergency room with a popliteal and left calf pain that has been worsening for 2 days. The echo Doppler revealed a popliteal and femoral deep vein thrombosis, and a left SFA PSA (15 × 18 mm) circulating with a 5-mm collar (Fig. 6A).
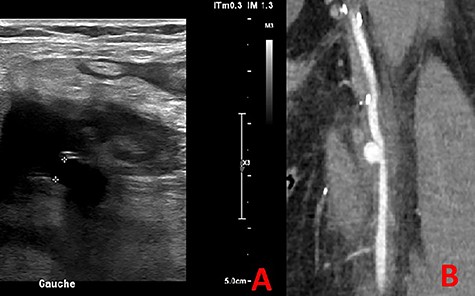
The CTA confirmed a PSA measuring 13 mm in diameter (Fig. 6B).
In view of the size of the PSA and the asymptomatic character, we decided to treat it by endovascular procedure.
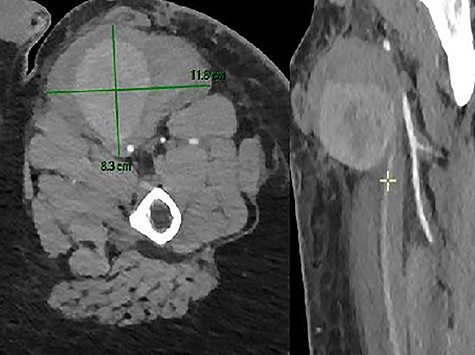
Unfortunately, in the meantime, he presented to the emergency room for a swelling of his left thigh since the morning. He had no pain. He was hemodynamically stable. His hemoglobin was 13 g/dl. We examined a large pulsatile mass of the left upper leg. The CTA showed a PSA (8.3 × 10.7 × 9 cm) of his left SFA and a hematoma with active bleeding on the anterior aspect of the left thigh, in close contact with the left SFA (Fig. 7).
The patient was operated as an emergency. A voluminous hematoma was observed involving muscle tissues in the anteromedial region of the thigh. Proximal control was obtained, the aneurysm incised and the distal control obtained with a fogarty catheter. The hematoma was evacuated and the PSA was resected. The anatomical structures are hardly recognizable and deformed due to the presence of this important hematoma. Our old venous bypass was not visualized, probably compressed and evacuated with the hematoma. The reconstruction performed by interposition of graft (Dacron 6 mm), with end-to-side anastomosis.
DISCUSSION
Spontaneous SFA PSA are extremely rare and rupture is yet more rare. (13-22) Only a very limited number of cases have been published in the literature. We conducted a chronological review of spontaneous SFA PSA cases without underlying atherosclerotic disease or notion of trauma (Table 1).
| Report . | Age (Y) . | Gender . | Imaging . | Laterality . | Size (mm) . | Treatment . |
|---|---|---|---|---|---|---|
| Case present first operation | 17 | M | US, CTA | Left, proximal | 67 × 50 × 80 | Surgical treatment |
| Case present second operation | 17 | M | CTA | Left, mid segment | 83 × 107 × 90 | Surgical treatment |
| Ugurlucan et al. [6] 2014 | 45 | M | US,CTA | Left, mid segment | 117 × 63 × 75 | Surgical treatment |
| Fukunaga et al. [7] 2013 | 77 | M | CTA | Right, proximal | N/A | Surgical treatment |
| Samara et al. [8] 2013 | 62 | F | US,MRA,A | Left, distal | 70x60 | Covered stent |
| Alsmady et al. [9] 2012 | 29 | M | CTA | Right, lower third | N/A | Surgical treatment |
| Kouvelas et al. [3] 2011 | 71 | F | CTA | Right, proximal | 100 | Surgical treatment |
| Siani et al. [5] 2008 | 86 | F | US,CTA,MRA,A | Left, mid segment | 40 | Covered stent |
| Lossef et al. [10] 2008 | 70 | M | A | Right SFA muscular branch | 2.5 | Spontaneous obliteration |
| Ramus et al. [11] 2007 | 74 | M | US,MRA,CA | Left, proximal | 40 and 50 (bilobed) | Covered stent |
| Goh et al. [12] 2004 | 15 | M | US,MRI,CA | Bilateral muscular SFA branches | N/A | Embolisation and surgical treatment |
| Lenartova et al. [2] 2003 | 82 | F | A | Right SFA muscular branch | N/A | Surgical treatment |
| Report . | Age (Y) . | Gender . | Imaging . | Laterality . | Size (mm) . | Treatment . |
|---|---|---|---|---|---|---|
| Case present first operation | 17 | M | US, CTA | Left, proximal | 67 × 50 × 80 | Surgical treatment |
| Case present second operation | 17 | M | CTA | Left, mid segment | 83 × 107 × 90 | Surgical treatment |
| Ugurlucan et al. [6] 2014 | 45 | M | US,CTA | Left, mid segment | 117 × 63 × 75 | Surgical treatment |
| Fukunaga et al. [7] 2013 | 77 | M | CTA | Right, proximal | N/A | Surgical treatment |
| Samara et al. [8] 2013 | 62 | F | US,MRA,A | Left, distal | 70x60 | Covered stent |
| Alsmady et al. [9] 2012 | 29 | M | CTA | Right, lower third | N/A | Surgical treatment |
| Kouvelas et al. [3] 2011 | 71 | F | CTA | Right, proximal | 100 | Surgical treatment |
| Siani et al. [5] 2008 | 86 | F | US,CTA,MRA,A | Left, mid segment | 40 | Covered stent |
| Lossef et al. [10] 2008 | 70 | M | A | Right SFA muscular branch | 2.5 | Spontaneous obliteration |
| Ramus et al. [11] 2007 | 74 | M | US,MRA,CA | Left, proximal | 40 and 50 (bilobed) | Covered stent |
| Goh et al. [12] 2004 | 15 | M | US,MRI,CA | Bilateral muscular SFA branches | N/A | Embolisation and surgical treatment |
| Lenartova et al. [2] 2003 | 82 | F | A | Right SFA muscular branch | N/A | Surgical treatment |
Abbreviations: US = ultrasound; MRI: magnetic resonance imaging; A = angiography; CIA = computed tomography angiogram; N/A = not available.
| Report . | Age (Y) . | Gender . | Imaging . | Laterality . | Size (mm) . | Treatment . |
|---|---|---|---|---|---|---|
| Case present first operation | 17 | M | US, CTA | Left, proximal | 67 × 50 × 80 | Surgical treatment |
| Case present second operation | 17 | M | CTA | Left, mid segment | 83 × 107 × 90 | Surgical treatment |
| Ugurlucan et al. [6] 2014 | 45 | M | US,CTA | Left, mid segment | 117 × 63 × 75 | Surgical treatment |
| Fukunaga et al. [7] 2013 | 77 | M | CTA | Right, proximal | N/A | Surgical treatment |
| Samara et al. [8] 2013 | 62 | F | US,MRA,A | Left, distal | 70x60 | Covered stent |
| Alsmady et al. [9] 2012 | 29 | M | CTA | Right, lower third | N/A | Surgical treatment |
| Kouvelas et al. [3] 2011 | 71 | F | CTA | Right, proximal | 100 | Surgical treatment |
| Siani et al. [5] 2008 | 86 | F | US,CTA,MRA,A | Left, mid segment | 40 | Covered stent |
| Lossef et al. [10] 2008 | 70 | M | A | Right SFA muscular branch | 2.5 | Spontaneous obliteration |
| Ramus et al. [11] 2007 | 74 | M | US,MRA,CA | Left, proximal | 40 and 50 (bilobed) | Covered stent |
| Goh et al. [12] 2004 | 15 | M | US,MRI,CA | Bilateral muscular SFA branches | N/A | Embolisation and surgical treatment |
| Lenartova et al. [2] 2003 | 82 | F | A | Right SFA muscular branch | N/A | Surgical treatment |
| Report . | Age (Y) . | Gender . | Imaging . | Laterality . | Size (mm) . | Treatment . |
|---|---|---|---|---|---|---|
| Case present first operation | 17 | M | US, CTA | Left, proximal | 67 × 50 × 80 | Surgical treatment |
| Case present second operation | 17 | M | CTA | Left, mid segment | 83 × 107 × 90 | Surgical treatment |
| Ugurlucan et al. [6] 2014 | 45 | M | US,CTA | Left, mid segment | 117 × 63 × 75 | Surgical treatment |
| Fukunaga et al. [7] 2013 | 77 | M | CTA | Right, proximal | N/A | Surgical treatment |
| Samara et al. [8] 2013 | 62 | F | US,MRA,A | Left, distal | 70x60 | Covered stent |
| Alsmady et al. [9] 2012 | 29 | M | CTA | Right, lower third | N/A | Surgical treatment |
| Kouvelas et al. [3] 2011 | 71 | F | CTA | Right, proximal | 100 | Surgical treatment |
| Siani et al. [5] 2008 | 86 | F | US,CTA,MRA,A | Left, mid segment | 40 | Covered stent |
| Lossef et al. [10] 2008 | 70 | M | A | Right SFA muscular branch | 2.5 | Spontaneous obliteration |
| Ramus et al. [11] 2007 | 74 | M | US,MRA,CA | Left, proximal | 40 and 50 (bilobed) | Covered stent |
| Goh et al. [12] 2004 | 15 | M | US,MRI,CA | Bilateral muscular SFA branches | N/A | Embolisation and surgical treatment |
| Lenartova et al. [2] 2003 | 82 | F | A | Right SFA muscular branch | N/A | Surgical treatment |
Abbreviations: US = ultrasound; MRI: magnetic resonance imaging; A = angiography; CIA = computed tomography angiogram; N/A = not available.
In the cases included in our table, none was related to traumatic shock, previous surgery or vascular access puncture site.
None of the patients had known atherosclerotic disease. Although no arthrosclerosis was described in any of the cases included in the supplementary examinations, nor described during surgery, nor found in the histopathology of the surgical specimen, it can be assumed that the cardiovascular factors present in some patients may have weakened their wall and consequently favored the PSA and subsequent development of the surrounding hematoma. Four of the cases included were at cardiovascular risk. Indeed, arterial hypertension was presented in the cases described by Fukunaga et al., Samara et al., Ramus et al.; and Lenovarta et al. The patients described by Samara et al.; and Lenovarta et al. also had diabetes. The one of Samara et al. had in addition hypercholesterolemia. And finally, the patient described by Ramus et al. also had chronic renal failure and warfarinized atrial fibrillation.
We also note that the average age of spontaneous PSAs is in the elderly, eight of the cases being older than 60 years and seven being older than 70 years. It can be assumed that the older a patient is, the more likely his arterial wall becomes fragile. No cases of large spontaneous PSA of our patient’size have been described under age 18 years. The case of Goh et al. involved small muscle branches of normal superficial femoral arteries. In our case, the PSA was spontaneous and giant, which is a very rare event considering his age.
In the case described by Urgulucan et al., the patient has Behcet's disease treated with immunosuppressive therapy, which could also explain a weakness of the arterial wall and consequently the appearance of a spontaneous PSA.
Only two cases are very similar to ours, Alsmady et al.; and Goh et al. who describe the occurrence of a spontaneous PSA in young patient without any cardiovascular factors. A notable difference with the case of Goh et al. remains the size of the aneurysm. In both cases, no genetic disease of the collagenosis type was found. The workup is still ongoing for our patient, although the first analyses were negative and he does not fit the morphotype of this type of disease.
A PSA occurring in unusual sites or spontaneously, especially in young people, should raise the possibility of vasculitis or connective tissue disease. A genetic workup should be requested. Currently, the mechanisms of spontaneous rupture of the SFA are still unknown.
It can be noted from the table that open surgical repair has traditionally been considered based on the patient's age and has been considered more desirable if the PSA did not cause significant hemodynamic or neurologic effects in the patient. Minimally invasive surgery did not necessarily depend on the size of the PSA. Indeed, Siana et al. successfully treated a spontaneous acute PSA of the ASF measuring 4 cm in diameter with a VIABAHN®-coated stent. Ramus et al. reported the use of a FLUENCY® Plus stent for the successful treatment of a 5- and 4-cm bilobed superficial femoral PSA. Samara et al. successfully treated a 7*6-cm PSA by endovascular technique. Limitation of endovascular technique consists of the inability to obtain cultures or biopsies in order to diagnose infection or congenital abnormality.
Various treatments, ranging from conservative to surgical, have been proposed and with the advances in endovascular repair, their utility has been reported. Unfortunately, there are no guidelines for the management of spontaneous PSAs. The optimal treatment is decided by the surgeon according to his experience and that of his center, the location of the PSA and the possibility of minimally invasive treatment. Therapeutic options for femoral PSA include open surgical repair, ultrasound-guided compression, ultrasound-guided thrombin injection, coil embolization and endovascular repair using stent-grafts [3].
Despite its rarity, we have various choices of treatments. However, open surgical repair has traditionally been considered the standard treatment for PSA [4]. Particularly in young patients, an appropriate approach is surgical exploration with hematoma evacuation and arterial repair by means of arterial suture, patch angioplasty or graft interposition [5].
In conclusion spontaneous PSAs of the SFA are extremely rare and no cases of giant PSA of the SFA in a minor patient have been published. In young people, the possibility of vasculitis or connective tissue disease should be considered. A genetic workup should be requested. We note that in the cases described in the literature, the majority of patients are older than 60 years and have cardiovascular risk factors. There are no guidelines for management. The optimal treatment is decided by the surgeon based to his or her experience, the location of the PSA and the possibility of minimally invasive treatment.
CONFLICT OF INTEREST STATEMENT
We report no conflicts of interest.
References
- pseudoaneurysm
- edema
- saphenous vein graft
- hemorrhage
- aneurysm
- hematoma
- emergency service, hospital
- femoral artery
- pain
- prostate-specific antigen
- reconstructive surgical procedures
- surgical procedures, operative
- tissue transplants
- wounds and injuries
- diagnostic imaging
- lower limb pain
- femoral artery, superficial
- rare diseases



