-
PDF
- Split View
-
Views
-
Cite
Cite
Yuko Komatsu, Tadashi Kawai, Shoko Miura, Yasunori Takeda, Hiroyuki Yamada, Rhabdomyosarcoma in the maxillary gingiva of a child patient, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab322, https://doi.org/10.1093/jscr/rjab322
Close - Share Icon Share
Abstract
Rhabdomyosarcoma (RMS) is a rare, rapidly growing and aggressive malignant neoplasm mainly affecting children. However, mean age at the diagnosis of patients with gingival RMS is 26.9 years. A 12-year-old girl presented to our clinic with a chief complaint of trismus. The examination findings indicated a malignant tumor in the left maxillary gingiva. We performed a biopsy of the tumor, and the histopathological diagnosis was RMS. We report a rare case of primary RMS of the maxillary gingiva in a child patient.
INTRODUCTION
Rhabdomyosarcoma (RMS) is a malignant neoplasm, which was first described by Weber in 1845 [1]. RMS is a rare, rapidly growing and aggressive malignant neoplasm that accounts for ~4–8% of cancer in children under 15 years of age. RMS mainly affects children (60%), and head and neck lesions account for 35–40% of cases. Oral lesions are uncommon, accounting for only 10–12% of all head and neck RMS. However, mean age at the diagnosis of patients with gingival RMS is 26.9 years [2]. Here, we report a case of RMS in the left maxillary gingiva of a 12-year-old girl.
CASE REPORT
A 12-year-old girl presented to our clinic with a chief complaint of trismus. A few months ago, she noticed a bulge in the left maxillary gingiva and trismus. There was no spontaneous pain, but pressure pain was felt. She was afebrile, had no history of trauma or medications. During her first medical examination at our clinic, she was 164 cm tall, weighed 54 kg. Extraoral examination revealed a slight bulge in the left cheek with countenance, right and left asymmetry and trismus. The range of mouth opening was 18 mm, and there were no swollen lymph nodes that I could feel in the neck. Intraoral findings revealed a neoplastic lesion extending from the left maxillary first molar to the maxillary tuberosity (Fig. 1A). It interfered with the occlusion on that side.
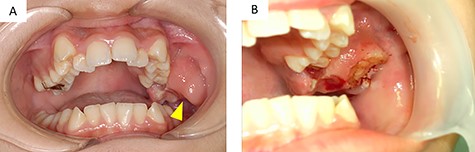
(A) intraoral findings of a suspected neoplastic lesion extending from the maxillary tuberosity to the left maxillary first molar at the time of initial examination (arrowhead); (B) intraoral findings at the time of biopsy; the sectioned surface was milky-white with enhancement characteristics
Panoramic radiography showed impaction of the left maxillary second molar and resorption of alveolar bone between the left maxillary molar area and the maxillary tuberosity (Fig. 2A).
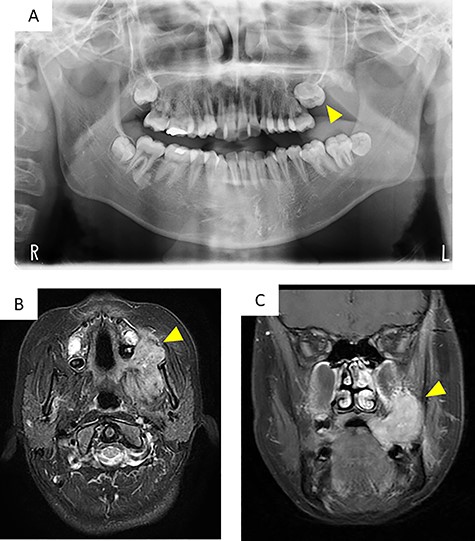
Imaging findings; (A) a panoramic radiograph showing impaction of the maxillary second molar and resorption of alveolar bone between the left maxillary molar area and the maxillary tuberosity (arrowhead); (B and C) contrast-enhanced T-1 weighted MRI showing a high signal in the mass extending from the left masticator space to the buccal space and the maxillary and mandibular alveolar regions (arrowhead)
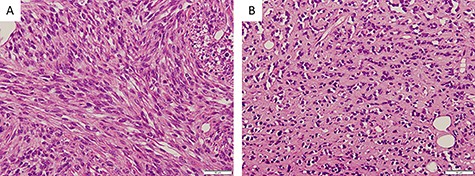
Histopathological findings; (A) proliferating oval to spindle-shaped cells, with hyperchromatic nuclei and high nuclear-cytoplasmic ratio, arranged in a fascicular pattern, and frequent mitotic figures (×200); (B) tumor cells arranged in small nests and pseudo-capillary pattern in the sclerotic collagenous stroma, in part (×200)
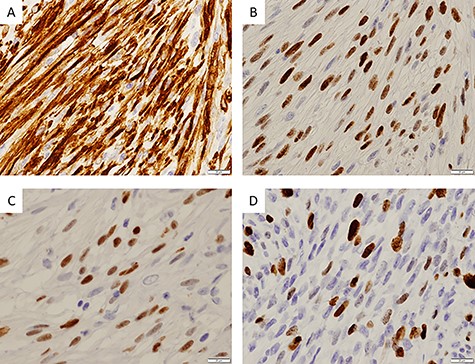
Immunohistochemical findings; (A) immunohistochemical staining showing positivity for desmin (×400); (B) myoD1 (×400); (C) myogenin (×400) and (D) ~30% of tumor cells were positive for Ki67 (×400)
| Maker . | Result . |
|---|---|
| MyoD1 | Positive |
| Desmin | Positive |
| Myogenin | Positive |
| Glial fibrillary acidic protein | Positive |
| Ki67 | 20–30%/HPF |
| AE1/AE3 | Negative |
| Epithelial membrane antigen | Negative |
| S100 | Negative |
| p53 | Negative |
| CD34 | Negative |
| SMA | Negative |
| Caldesmon | Negative |
| Myoglobin | Negative |
| Maker . | Result . |
|---|---|
| MyoD1 | Positive |
| Desmin | Positive |
| Myogenin | Positive |
| Glial fibrillary acidic protein | Positive |
| Ki67 | 20–30%/HPF |
| AE1/AE3 | Negative |
| Epithelial membrane antigen | Negative |
| S100 | Negative |
| p53 | Negative |
| CD34 | Negative |
| SMA | Negative |
| Caldesmon | Negative |
| Myoglobin | Negative |
| Maker . | Result . |
|---|---|
| MyoD1 | Positive |
| Desmin | Positive |
| Myogenin | Positive |
| Glial fibrillary acidic protein | Positive |
| Ki67 | 20–30%/HPF |
| AE1/AE3 | Negative |
| Epithelial membrane antigen | Negative |
| S100 | Negative |
| p53 | Negative |
| CD34 | Negative |
| SMA | Negative |
| Caldesmon | Negative |
| Myoglobin | Negative |
| Maker . | Result . |
|---|---|
| MyoD1 | Positive |
| Desmin | Positive |
| Myogenin | Positive |
| Glial fibrillary acidic protein | Positive |
| Ki67 | 20–30%/HPF |
| AE1/AE3 | Negative |
| Epithelial membrane antigen | Negative |
| S100 | Negative |
| p53 | Negative |
| CD34 | Negative |
| SMA | Negative |
| Caldesmon | Negative |
| Myoglobin | Negative |
Computed tomography (CT) images showed a mass that extended from the left masticator space to the buccal space and the alveolar regions of the maxilla and mandible and pressure resorption of the anterior border of ramus, rear wall of the maxillary sinus and lateral plate of the pterygoid process. Involvement of the maxillary sinus was also suspected. Contrast-enhanced T1-weighted magnetic resonance imaging (MRI) showed a high signal in a range similar to CT (Fig. 2B and C). Contrast-enhanced effects in the left cervical and retropharyngeal (Rouviere) lymph nodes were also detected. 18F-fluorodeoxyglucose (18FDG)-positron emission tomography/CT showed a mass measuring 63 × 38 × 45 mm (long axis × minor axis × height) located in the left maxillary gingiva, accompanied by abnormal accumulation of 18FDG (standardized uptake value [SUV] max, 8.3), and left cervical lymphadenopathy, accompanied by abnormal accumulation of 18FDG (SUVmax, 41.72), which was suspected as metastasis. Abnormal accumulation was not observed in the other organs.
The differential diagnosis for a malignant tumor of oral soft tissue includes squamous cell carcinoma, sarcoma and salivary gland malignant tumor. Ameloblastoma is considered in the differential diagnosis for a maxillary tumor with trismus. Ameloblastoma is a benign tumor that presents as a bulge on the jawbone and causes bone resorption. It has included extraosseous/peripheral ameloblastoma [3].
Three days after the first medical examination, a biopsy of the lesion was performed in the left maxillary gingiva with the consent of the patient and parents to obtain a definitive diagnosis. The sectioned surface was milky-white with enhancing characteristics (Fig. 1B). Histopathological examination showed proliferation of oval to spindle-shaped cells, with hyperchromatic nuclei and a high nuclear-cytoplasmic ratio, arranged in a fascicular pattern (Fig. 3A). Mitotic figures were frequently observed. Focally, the tumor cells were arranged in small nests and in a pseudo-capillary pattern in the sclerotic collagenous stroma (Fig. 3B). Immunohistochemical examination revealed that the tumor cells were positive for desmin, myoD1, myogenin, mammary serum antigen and glial fibrillary acidic protein (Fig. 4A–C). The Ki67 proliferation index was ~30% per HPF (Fig. 4D). The details of immunostaining are shown in Table 1. The patient was diagnosed with RMS classified as Group III according to the Intergroup Rhabdomyosarcoma Study (IRS) grouping system [4]. The left maxillary gingiva RMS was treated via proton beam irradiation at 59.4 Gy and with vacuum-assisted wound closure therapy with 14 cycles of vincristine, actinomycin D and cyclophosphamide. After chemoradiotherapy, a complete response was documented via image evaluation.
Cases of oral cavity RMS in patients under 20 years of age, including the present case, within the past decade (2011–21)
| Author . | Year . | Age . | Sex . | Part . | Major axis (cm) . | Chief complaint . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| Miloglu et al. [5] | 2011 | 13 | Girl | Buccal mucosa | NA | Swelling | Infosfamid + vincristine + actinomysin-D After 6 months, tumor was growth: carboplatin + epirubisin + vincristin, RT (54 Gy) | Death |
| Peter et al. [6] | 2017 | 7 | Boy | Mandible | 7.5 | Swelling | Vincristine + actinomycine + cyclophosphamide, RT (36 Gy), operation | No recurrence or metastasis |
| Mclnturff et al. [7] | 2017 | 19 | Girl | Buccal mucosa | 2.8 | Swelling | Referral to other hospital | NA |
| Shrutha et al. [8] | 2015 | 1 | Boy | Maxilla | ~6 | Swelling | Vincristine + actinomycine + cyclophosphamide + dexamethosane | Death |
| Alfazaz et al. [9] | 2019 | 14 | Boy | Palate | ~6 | Dysphonia, dysphagia and pain | Operation and adjuvant chemotherapy (NA) | Recurrence and metastases (pulmonary and lymph nodes) |
| Datta et al. [10] | 2016 | 17 | Girl | Buccal mucosa | ~3 | Swelling | Referral to other hospital | NA |
| This case | 2021 | 12 | Girl | Maxilla | 6.3 | Trismus | Vincristine + actinomycine + cyclophosphamide, RT (59.4 Gy) | Follow-up |
| Author . | Year . | Age . | Sex . | Part . | Major axis (cm) . | Chief complaint . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| Miloglu et al. [5] | 2011 | 13 | Girl | Buccal mucosa | NA | Swelling | Infosfamid + vincristine + actinomysin-D After 6 months, tumor was growth: carboplatin + epirubisin + vincristin, RT (54 Gy) | Death |
| Peter et al. [6] | 2017 | 7 | Boy | Mandible | 7.5 | Swelling | Vincristine + actinomycine + cyclophosphamide, RT (36 Gy), operation | No recurrence or metastasis |
| Mclnturff et al. [7] | 2017 | 19 | Girl | Buccal mucosa | 2.8 | Swelling | Referral to other hospital | NA |
| Shrutha et al. [8] | 2015 | 1 | Boy | Maxilla | ~6 | Swelling | Vincristine + actinomycine + cyclophosphamide + dexamethosane | Death |
| Alfazaz et al. [9] | 2019 | 14 | Boy | Palate | ~6 | Dysphonia, dysphagia and pain | Operation and adjuvant chemotherapy (NA) | Recurrence and metastases (pulmonary and lymph nodes) |
| Datta et al. [10] | 2016 | 17 | Girl | Buccal mucosa | ~3 | Swelling | Referral to other hospital | NA |
| This case | 2021 | 12 | Girl | Maxilla | 6.3 | Trismus | Vincristine + actinomycine + cyclophosphamide, RT (59.4 Gy) | Follow-up |
RT, radiation; NA, not available.
Cases of oral cavity RMS in patients under 20 years of age, including the present case, within the past decade (2011–21)
| Author . | Year . | Age . | Sex . | Part . | Major axis (cm) . | Chief complaint . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| Miloglu et al. [5] | 2011 | 13 | Girl | Buccal mucosa | NA | Swelling | Infosfamid + vincristine + actinomysin-D After 6 months, tumor was growth: carboplatin + epirubisin + vincristin, RT (54 Gy) | Death |
| Peter et al. [6] | 2017 | 7 | Boy | Mandible | 7.5 | Swelling | Vincristine + actinomycine + cyclophosphamide, RT (36 Gy), operation | No recurrence or metastasis |
| Mclnturff et al. [7] | 2017 | 19 | Girl | Buccal mucosa | 2.8 | Swelling | Referral to other hospital | NA |
| Shrutha et al. [8] | 2015 | 1 | Boy | Maxilla | ~6 | Swelling | Vincristine + actinomycine + cyclophosphamide + dexamethosane | Death |
| Alfazaz et al. [9] | 2019 | 14 | Boy | Palate | ~6 | Dysphonia, dysphagia and pain | Operation and adjuvant chemotherapy (NA) | Recurrence and metastases (pulmonary and lymph nodes) |
| Datta et al. [10] | 2016 | 17 | Girl | Buccal mucosa | ~3 | Swelling | Referral to other hospital | NA |
| This case | 2021 | 12 | Girl | Maxilla | 6.3 | Trismus | Vincristine + actinomycine + cyclophosphamide, RT (59.4 Gy) | Follow-up |
| Author . | Year . | Age . | Sex . | Part . | Major axis (cm) . | Chief complaint . | Treatment . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| Miloglu et al. [5] | 2011 | 13 | Girl | Buccal mucosa | NA | Swelling | Infosfamid + vincristine + actinomysin-D After 6 months, tumor was growth: carboplatin + epirubisin + vincristin, RT (54 Gy) | Death |
| Peter et al. [6] | 2017 | 7 | Boy | Mandible | 7.5 | Swelling | Vincristine + actinomycine + cyclophosphamide, RT (36 Gy), operation | No recurrence or metastasis |
| Mclnturff et al. [7] | 2017 | 19 | Girl | Buccal mucosa | 2.8 | Swelling | Referral to other hospital | NA |
| Shrutha et al. [8] | 2015 | 1 | Boy | Maxilla | ~6 | Swelling | Vincristine + actinomycine + cyclophosphamide + dexamethosane | Death |
| Alfazaz et al. [9] | 2019 | 14 | Boy | Palate | ~6 | Dysphonia, dysphagia and pain | Operation and adjuvant chemotherapy (NA) | Recurrence and metastases (pulmonary and lymph nodes) |
| Datta et al. [10] | 2016 | 17 | Girl | Buccal mucosa | ~3 | Swelling | Referral to other hospital | NA |
| This case | 2021 | 12 | Girl | Maxilla | 6.3 | Trismus | Vincristine + actinomycine + cyclophosphamide, RT (59.4 Gy) | Follow-up |
RT, radiation; NA, not available.
DISCUSSION
RMS mainly affects children (60%). However, mean age of patients at diagnosis with gingival RMS is 26.9 years [2]. Thus, most gingival RMS case reports have featured adult patients despite RMS being more common in children. This case was unique because RMS was observed in a 12-year-old patient.
Seven cases of oral cavity RMS have been reported in patients under 20 years of age, including the present case, within the past decade (2011–21) [5–10] (Table 2). According to a previous report, the common signs and symptoms of oral RMS include tooth mobility, paresthesia, trismus and cervical lymphadenopathy [2]. These symptoms are manifestations of the growing tumor. In our case, there was trismus and cervical lymphadenopathy. However, these symptoms are not specific to RMS but could be evident in any malignant tumor. Therefore, a pathologic diagnosis using immunohistochemistry is necessary. If a malignant tumor that cannot be identified by clinical findings is suspected, a biopsy should be performed at an early stage. In this case, CT and contrast-enhanced MRI were performed early, and a biopsy was performed 3 days after the initial examination.
We report a rare case of primary RMS of the maxillary gingiva in a pediatric patient. In this case, the RMS was diagnosed early and appropriate treatment was initiated. The absence of metastasis to other organs might be one of the reasons why the prognosis of this case was good.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



