-
PDF
- Split View
-
Views
-
Cite
Cite
Kenneth Y Y Kok, Edwin C C Lim, Kimura’s disease: a rare cause of chronic neck lymphadenopathy, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab318, https://doi.org/10.1093/jscr/rjab318
Close - Share Icon Share
Abstract
Kimura’s disease is a rare chronic inflammatory disorder of unknown etiology which typically presents with subcutaneous nodules in the head and neck region and is frequently associated with regional lymphadenopathy or salivary gland enlargement. Peripheral blood eosinophilia and elevated serum immunoglobulin E levels are constant features of the disease. We present herein a 31-year-old male patient who presented with chronic neck lymphadenopathy. Kimura’s disease was diagnosed on fine needle aspiration cytology, the patient initially decided not to have further intervention. He presented 6 years later with lymphadenopathy and was treated with surgery. The diagnosis of Kimura’s disease was confirmed on histopathology. This patient had the disease for 6 years and did not have the typical features of peripheral eosinophilia and raise serum IgE level.
INTRODUCTION
Kimura’s disease first described by Kim and Szeto in 1937 is a rare chronic inflammatory disorder of unknown etiology [1]. It was later named Kimura’s disease in 1948 after Kimura et al. described in detail the pathological features of the disease [2]. Allergic reaction, Candida infection, arthropod bite, eosinophil and immunoglobulin E (IgE) deregulation and altered systemic immune mediation have all been postulated as possible causes of the disease [3].
Kimura’s disease typically presents with subcutaneous nodules in the head and neck region and is frequently associated with regional lymphadenopathy or salivary gland enlargement [4]. Peripheral blood eosinophilia and elevated serum IgE levels are constant features of the disease [5]. The disease is generally reported in young adults between the ages of 20 and 40 years; men being more commonly affected than women, with a ratio of 3:1 [6, 7]. It is commonly seen in Asians, but has also been reported sporadically in other races [7]. Coexisting renal disease has been reported in Kimura’s disease [7]. The diagnosis of Kimura’s disease is often difficult, and a pathological study of the mass is often necessary to confirm the diagnosis.
There is no consensus on the definitive treatment for Kimura’s disease; with surgery, thalidomide, cyclosporine, interferon-α, omalizumab, corticosteroids and radiotherapy have all been recommended as treatment modalities [8, 9]. Recurrence rates of 62% have been reported [10]. We report herein a case of Kimura’s disease presented with chronic neck lymphadenopathy treated with surgery.
CASE REPORT
A 31-year-old male of South East Asian ethnicity presented with a painless swelling in left side of his neck which had been increasing in size for 6 months. He had no constitutional symptoms, weight loss, trauma or discharge from the swelling. There were no other swellings in the neck region. He had presented with the same swelling to another hospital 6 years ago and underwent some investigations.
Clinical examination revealed a 2.5 cm × 1.5 cm swelling in the left posterior triangle of the neck, posterior to the border of lower third of the left sternocleidomastoid muscle. The swelling was soft to firm in consistency, non-tender and mobile with normal overlying skin. There were no other subcutaneous nodules, swellings or lymphadenopathy in the head and neck region.
At his initial presentation 6 years ago, laboratory examination showed WBC of 6.9 × 109/l (normal 3.6–10.2 × 109/l); eosinophil 7.8% (normal 0.8–8.1%); eosinophil count 0.5 × 109/l (normal 0–0.5 × 109/l), and serum IgE 0.02 × 109/l (normal 0–0.04 × 109/l). Fine needle aspiration cytology (FNAC) of the swelling suggested Kimura’s disease. The patient was informed of the diagnosis and the benign nature of the swelling, and he decided not to have any further intervention.
Because of the recent increase in the size of the swelling, the patient presented to our clinic and agreed to undergo excision of the swelling. A well-circumscribed swelling measuring 2.5 cm × 1.5 cm which was soft to firm in consistency was completely excised (Fig. 1). Histopathological examination showed sections of lymph node with florid follicular and germinal center hyperplasia (Fig. 2), and paracortical expansion by plasma cells, small lymphocytes and mast cells (Fig. 3). Marked eosinophilic infiltration with focal micro-abscess formation was present (Fig. 4). The features were consistent with Kimura’s disease. There was no evidence of malignancy. At 6 months follow-up, the patient had no evidence of recurrence.
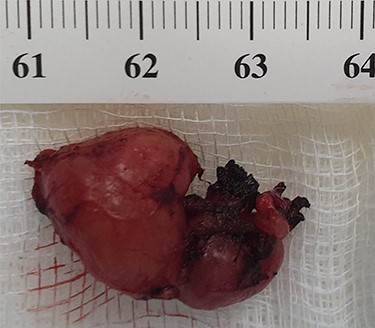
Well-circumscribed and lobulated lymph node measuring 2.5 cm × 1.5 cm.
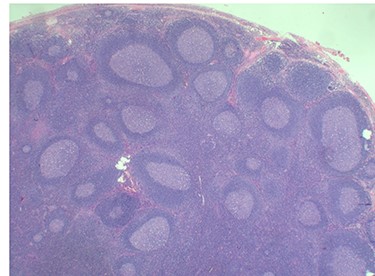
Histopathology of the lymph node showing follicular and germinal center hyperplasia. Haematoxylin and Eosin stain. Magnification ×1.2.
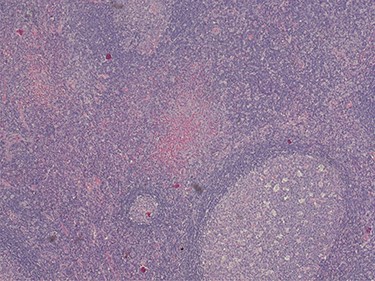
Histopathology of the lymph node showing paracortical expansion by plasma cells, small lymphocytes and mast cells, and areas containing eosinophilic micro-abscesses (accumulation of inter-follicular eosinophils). Haematoxylin and Eosin stain. Magnification ×5.
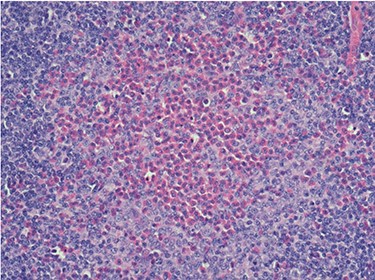
Histopathology of the lymph node showing an eosinophilic micro-abscess. Haematoxylin and Eosin stain. Magnification ×20.
DISCUSSION
Our patient’s presentation of Kimura’s disease is unusual in a number of ways. First, he presented with only neck lymphadenopathy. Typically, patients with Kimura’s disease present with subcutaneous nodules in the head and neck region with regional lymphadenopathy with or without salivary gland involvement [4]. Its presentation primarily as neck lymphadenopathy without subcutaneous skin or salivary gland involvement is exceedingly rare [11].
Second, Kimura’s disease had a long chronicity in our patient. The patient was informed of the diagnosis after FNAC at his initial consultation. Kimura’s disease has been known to be a benign and indolent condition [12]. Our patient had the disease for 6 years before his latest presentation.
Third, Kimura’s disease with normal peripheral eosinophil and serum IgE levels is very rare. Diagnosis of Kimura disease can be challenging and the most consistent laboratory findings have been peripheral eosinophilia and elevated serum IgE levels [5]. There are only a small number of reported cases of Kimura’s disease with normal peripheral eosinophil and/or serum IgE levels [12].
Some authors were able to diagnose Kimura’s disease on FNAC [13], whereas others have reported the swellings as lymphadenitis or malignancies [14, 15]. The characteristic features of Kimura’s disease on FNAC are the presence of significant numbers of eosinophils in a background of lymphoid cells, with fragments of collagenous tissue, endothelial cells and occasional polykaryocytes [14, 15].
The prominent histopathological features of Kimura’s disease are lymphoid tissue presenting with germinal center hyperplasia surrounded by hyperplastic vascular structures. Lymphoid follicles are composed of germinal centers of variable dimensions containing eosinophil infiltrates of various degrees and focal eosinophil micro-abscesses. Marked vascular hyperplasia and fibrosis are also present. No atypical cells are observed [13–15].
Renal involvement has been reported in patients with Kimura’s disease, with an incidence ranging from 10 to 60% [7]. Nephrotic syndrome was seen 10–12% of these patients, others may manifest as membranous glomerulonephritis, minimal change glomerulonephritis, diffuse proliferative glomerulonephritis or mesangial glomerulonephritis [7, 11].
The definitive treatment for Kimura’s disease remains controversial [8, 9]. Surgery has been considered as the gold standard treatment modality. In cases where the disease involves the subcutaneous tissue without well-defined margins, a combination of surgery with postoperative systemic corticosteroids and low-dose radiotherapy have been recommended [12, 13], as recurrence rate of up to 62% have been reported in these cases with surgery alone [10].
CONCLUSION
Kimura’s disease that has an indolence course can present rarely as neck lymphadenopathy of long chronicity, and it may not have the typical features of peripheral eosinophilia and raised serum IgE level. The diagnosis can be difficult and misleading, and FNAC with its typical features may be helpful in providing the initial diagnosis. The definitive diagnosis can only be confirmed with histopathological examination of the excised swelling. In our patient surgery alone was an adequate treatment and follow-up at 6 months showed no recurrences.
AUTHORS’ CONTRIBUTIONS
Both authors made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed on the journal to which the article will be submitted; gave final approval of the version to be submitted for publication; and agree to be accountable for all aspects of the work.
FUNDING
This research did not receive any specific grant or funding.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
References
- neck
- subcutaneous nodules
- fine needle aspiration biopsy for cytology
- surgical procedures, operative
- diagnosis
- immunoglobulin e
- surgery specialty
- kimura disease
- inflammatory disorders
- head and neck
- salivary gland hypertrophy
- causality
- histopathology tests
- lymphadenopathy
- peripheral blood eosinophilia



