-
PDF
- Split View
-
Views
-
Cite
Cite
Amr Elgazar, Ahmed K Awad, Debvarsha Mnadal, Merihan A Elbadawy, Sheref A Elseidy, Synchronous breast invasive ductal carcinoma and clear cell renal carcinoma: case report and a review of literature, Journal of Surgical Case Reports, Volume 2021, Issue 7, July 2021, rjab317, https://doi.org/10.1093/jscr/rjab317
Close - Share Icon Share
Abstract
Multiple primary tumors' incidence is rare, yet more rare is the incidence of multiple primary malignant tumors. Co-occurring tumors can be divided into synchronous and non-synchronous. Synchronous tumors are those tumors that present within a period not >6 months from each other. To define synchronous malignant tumors: metastasis should not be present, both tumors have to show criteria of malignancy, and they should differ pathologically from each other. Breast cancer is the most common tumor to be associated with other primaries especially; colorectal cancer, endometrial and ovarian cancer, yet the occurrence of invasive ductal carcinoma with clear cell renal cancer is uncommon. In our case, we present a 59-year-old female with invasive ductal carcinoma and clear cell renal carcinoma.
INTRODUCTION
The discovery of patients with multiple primary malignant tumors is increasing nowadays; mostly due to the advances in screening protocols more tumors are discovered incidentally and due to growing awareness of preventive care [1]. Synchronous tumors are those tumors that present within a period not >6 months from each other [2]. Although being reported during the past decade more frequently, multiple primary malignancy mechanisms have not yet been clarified. Multiple mechanisms have been proposed in the pathogenesis including immune, hereditary and environmental factors such as chemicals, viruses, chemotherapeutic regimens and ionizing radiation [2]. Breast cancer is the most common tumor to be associated with other primaries especially; colorectal cancer, endometrial and ovarian cancer, yet the occurrence of invasive ductal carcinoma with clear cell renal cancer is uncommon. We present a rare case of synchronous breast cancer and renal cell carcinoma which was discovered incidentally during the routine metastatic workup.
CASE REPORT
A 59-year-old female patient presented to our outpatient clinic with a palpable mass in her left breast. The patient had no family history of neither breast cancer, endometrial nor ovarian cancer and no comorbidities except for hypertension which was controlled. On examination the mass was suspicious and hard inconsistency; a mammogram was done, and it revealed a BIRADS (4) breast mass 13.5 × 8.5 mm at 3 o’clock position (Fig. 1).
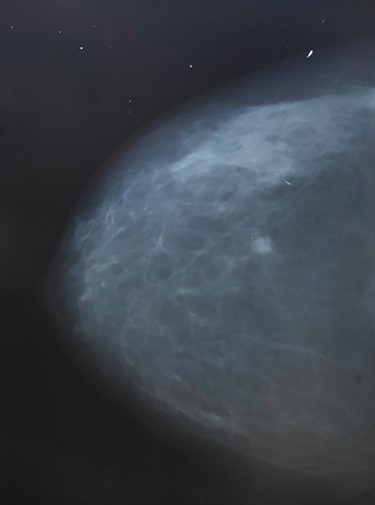
Mammogram shows braids 4 suspicious mass at left upper quadrant (lt breast).
Fine needle aspiration cytology (FNAC) was done and it showed moderately differentiated invasive ductal carcinoma. Metastatic workup was requested including Chest X-rays, computed tomography (CT) scans and positron emission tomography (PET) scans, and were all free. Preoperative laboratory works up were all within normal ranges; CA.15.3 was 16 U/ml, creatinine: 1.1 mg/dl and HB; 10 mg/dl.
Preoperative laboratory examination was all within normal ranges; CA.15.3 was 16 U/ml, creatinine: 1.1 mg/dl and HB; 10 mg/dl. The patient went for conservative breast surgery in the form of wide local excision with intraoperative frozen section examination which confirms the malignant nature of the mass and ensures that there was no invasion at the surgical margins, so we proceed also to Levels 1 and 2 axillary lymph nodal clearance. The mass was excised, and the patient was discharged on the next day with no postoperative complications.
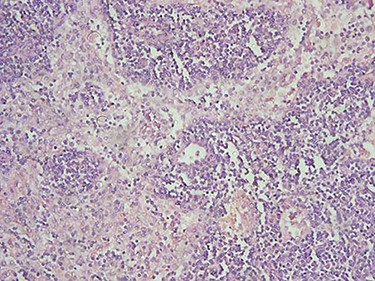
Histopathological paraffin examination confirmed that it was invasive ductal carcinoma (IDC) grad 2, T1, with no intraductal component, free surgical margins and no axillary lymph nodal metastasis LN: 0/15. There were neither tumor emboli nor tumor necrosis and the mitotic index was low (Fig. 2). Immunohistochemistry showed that the mass is positive for Estrogen (Score8/8), positive for Progesterone (score8/8) and negative for HER2 protein overexpression (Score0/8) (Fig. 3).
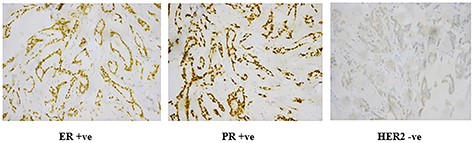
Immunohistochemistry of IDC; processed in Ventana and stained by rabbit monoclonal antibodies using DAB as chromogen and hematoxylin as counterstain; (A) ER receptors (+ve nuclear staining by chromogen); (B) PR receptors (+ve nuclear staining by chromogen); (C) HER2 (−ve cell membrane staining).
She was scheduled to take 30 sessions of radiotherapy and then to be on hormonal therapy in the form of Tamoxifen 20 mg/day for 5 years. During the last post-operative follow-up—3 months later—the patient complains of right hypochondrial pain; furthermore, the patient was scheduled for a multi-slice Triphasic Pelvi-abdominal CT which demonstrated the presence of a highly vascular renal mass with enhancement in the arterial phase and fading out in the later phases of examination suggestive for malignancy with patent inferior vena cava (IVC) and renal vein. There was no peri-renal or abdominal lymphadenopathy (Fig. 4).
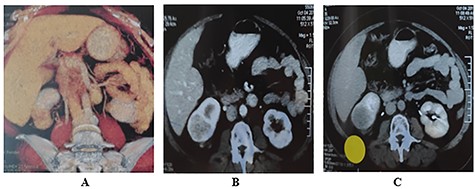
Triphasic multi-slice pelvi-abdominal CT scan showing exophytic mass at the lower pole of Rt Kidney; (A) Colored 2D CT reconstruction; (B) Enhancement of tumor in the arterial phase; (C) Fading out of the contrast in the venous phase.
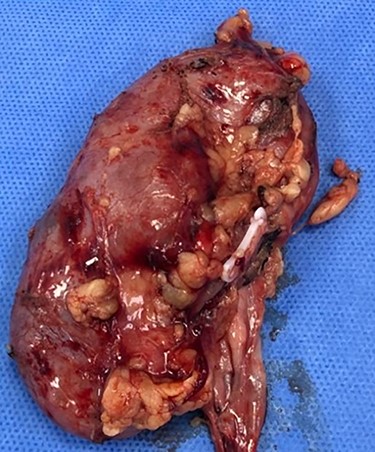
Consequently, the patient was scheduled for Rt radical nephrectomy which was done through Rt transverse paramedian incision. The renal specimen is shown in Fig. 5. The postoperative course went smoothly except for the wound infection which was managed conservatively. Histopathological examination showed that it was clear cell renal carcinoma, grade 2, T3a, free renal vein and lymph nodes were also free from any metastatic deposits (Score0/8; Fig. 6).
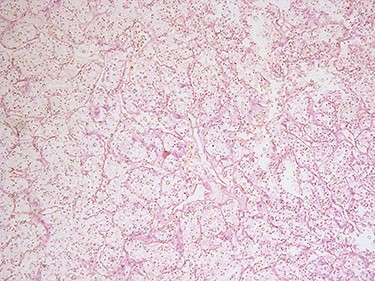
H&E microscopic appearance of clear cell renal carcinoma (100 times magnification).
The patient was followed with PET-CT after 1 year and then a mammogram every year and pelvi-abdominal U/S every 6 months and there was not any evidence of regional recurrence or distant metastasis at 5 years follow-up.
DISCUSSION
In patients with malignant tumors, the incidence of more than one primary tumor varies between 2.4 and 8%, up to 17% [3]. Several epidemiological factors were investigated for the etiology of more than one primary tumor. Genetic mutation disorders, hormonal factors, alcohol, tobacco smoking, infections and occupational exposure hazards were the most common causes for this presentation [3]. Among all primary tumors, breast cancer is the most common primary tumor to be associated with other multiple primaries [4].
Criteria to define Synchronous malignant tumors include: (i) presentation of two or more tumors simultaneously or within 6 months from each other [5]; (ii) probability of metastasis should be excluded (iii) and both tumors must present malignancy differing pathologically from each other [6]. Estrogen -according to many studies [7, 8] is responsible for the development of many cancers especially breast, endometrium and ovarian cancers. Many theories implicate that estrogen plays alongside genetic landscape a regulatory role in the development of multiple carcinomas, estrogen signaling is characterized in tumor cells enabling cell proliferation via activation of tumor-promoting transcriptional regulation of genes. However, very little is known about the definitive role of estrogen in the modulation of synchronous tumors and further studies need to conclude [7].
On reviewing the literature, the occurrence of synchronous IDC and renal cell carcinoma (RCC) is uncommon. However, in a retrospective-based analysis of 72 Chinese patients, Jiao et al. recorded 16 (22.2%) cases of synchronous primary malignant tumors of which 8 cases were breast and renal cancer [9]. Arjunan and Ravi, et al. also reported a case of synchronous IDC and papillary renal cell cancer [10]. Only reported once before in the literature, a case similar to ours in which the renal mass was also incidentally discovered during the metastatic workup of the breast lesion [11]. Meticulous histopathological examination of the specimens is vital to rule out metastasis as in some rare cases we could have multiple primaries that send off metastatic deposits to the core of another primary tumor leading to a complete change in the treatment plan [12, 13].
In our case, the renal tumor was incidentally discovered during the metastatic workup of the breast lesion. There were no presented clinical manifestations of renal tumor that would concern the patient to seek medical advice. Although the patient was operated on at two different surgeries, due to the lack of data detected by ultrasound of the renal tumor and low suspicion of malignancy as it is a rare entity to present as a synchronous tumor or as a metastasis with breast cancer, we emphasize on the value of guided biopsy from the tumor whenever definite diagnosis can’t be reached.
CONCLUSION
The synchronous occurrence of breast and renal cell carcinoma is uncommon, has not been reported in literature except for two occasions. With the advances in screening and follow-up protocols, the early detection of additional tumors and understanding the different aspects of correlation between these tumors will be plausible, yet the finding of a new primary malignant tumor should not be surprising.
References
Elseidy SA, Alkader AAAA, Naserallah HH, Awad AK.



