-
PDF
- Split View
-
Views
-
Cite
Cite
Takahiro Sanada, Hajime Wada, Hirotaka Sato, Wakako Shirai, Manabu Kinoshita, Naoki Tokumitsu, Carotid artery stenting assisted with intravascular ultrasonography for isolated spontaneous common carotid artery dissection, Journal of Surgical Case Reports, Volume 2021, Issue 6, June 2021, rjab232, https://doi.org/10.1093/jscr/rjab232
Close - Share Icon Share
Abstract
Isolated spontaneous common carotid artery (CCA) dissection is extremely rare. Moreover, only a few case reports for isolated spontaneous CCA dissection treated with carotid artery stenting (CAS) can be found so far. Here, the authors report a case where intravascular ultrasonography (IVUS) provided valuable information about lesion evaluation, stent selection and stent placement during CAS for isolated CCA dissection. A 69-year-old male was diagnosed with an isolated spontaneous left CCA dissection. CAS assisted with IVUS was performed to prevent further dissection and cerebral infarction recurrence. To the best of our knowledge, this is the first case report of an isolated spontaneous CCA dissection treated with CAS assisted by IVUS. CAS assisted by IVUS may be an effective treatment option to prevent intraoperative complications and further stroke recurrence for isolated spontaneous CCA dissection.
INTRODUCTION
Cervical artery dissection accounts for 1–2% of all ischemic stroke [1]. Although we often encounter internal carotid artery dissection or vertebral artery, common carotid artery (CCA) dissection is estimated to account for <1% of all cervical artery dissection and is a rare cause of ischemic stroke [2, 3]. Aortic dissection frequently accompanies CCA dissection, and spontaneous CCA dissection is extremely rare [4], except for those caused by neck trauma or endovascular interventions [5].
Although there is no evidence-based guideline for treating this condition and endovascular management is proffered for extracranial artery dissection thanks to its lower complication rate and immediate vascular reconstruction [6], only a few case reports for isolated spontaneous CCA dissection treated with carotid artery stenting (CAS) can be found so far [7, 8], Intravascular ultrasonography (IVUS) can also provide valuable information about lesion evaluation, stent selection and stent placement during treatment for dissection lesions in the ICA, vertebral artery and CCA dissection developing from aortic artery dissection [9–11]. However, CAS for isolated CCA dissection assisted by IVUS has not been reported yet. Here, we report a case of an isolated spontaneous CCA dissection treated with CAS assisted by IVUS.
CASE REPORT
A 69-year-old male visited the emergency department presenting right hemiplegia and total aphasia. The final safety confirmation was 9-h ago. The National Institutes of Health Stroke Scale score was 10. Diffusion weighted imaging (DWI) in magnetic resonance imaging showed high signal intensity in the left frontal lobe and basal ganglia. Magnetic resonance angiography (MRA) revealed a left CCA occlusion with collateral flow via anterior communicating and posterior communicating arteries (Fig. 1A). Computed tomography angiography (CTA) scanning also showed left CCA occlusion without aortic dissection (Fig. 1B). We could not find any clinically significant problem of the patients except atrial fibrillation detected on an electrical cardiogram. We initiated anticoagulant treatment based on finding chronic atrial fibrillation.
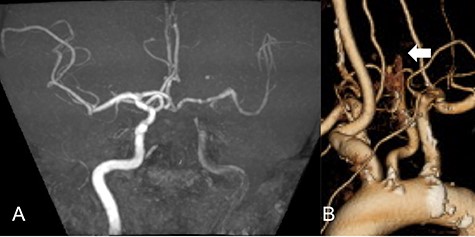
MRA demonstrated left CCA occlusion with collateral flow via anterior and posterior communicating arteries (A). CTA showed left CCA occlusion without aortic dissection (white arrow: B).
Repeated DWI images showed no significant expansion of the infarction, and complete recanalization of the left CCA was confirmed on MRA 2 weeks after initial onset (Fig. 2A). CTA revealed an irregular arterial wall and pseudo-lumen located in the CCA, extending to the bifurcation, suggesting an isolated left CCA dissection without the dissection extending to the aorta (Fig. 2B).
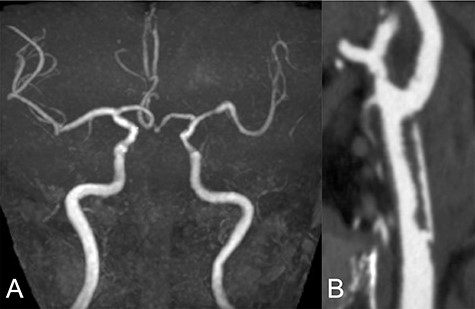
Two weeks after stroke onset, MRA revealed a complete recanalization of the left CCA (A). CTA also showed an irregular vessel wall and a pseudo-lumen located starting at the left CCA extending to the carotid bifurcation (B).
We performed CAS and started dual antiplatelet therapy 27 days after stroke onset to prevent further dissection and cerebral infarction recurrence. The dissection started 1.5-cm below the carotid bifurcation (Fig. 3A). An 8 fr guiding catheter (FlowGate2 Balloon Guide Catheter, Stryker, Fremont, CA, USA) was positioned at the proximal portion of the left CCA with a micro-guidewire (ASAHI CHIKAI Black, Asahi-Intecc, Nagoya, Aichi, Japan) crossing the dissected lesion. A distal protection device (SpiderFx Embolic Protection Device, Medtronic, Minneapolis, MN, USA) was deployed at the petrous portion of the ICA. IVUS was first advanced into the petrous ICA, and vessel wall imaging was performed by slowly withdrawing the device. IVUS imaging confirmed the existence of the dissection’s pseudo-lumen starting 1-cm proximal to the carotid bifurcation (Fig. 3C). IVUS provided the ICA and CCA diameters, which helped decide the most suitable stent (Fig. 3B and C). To adequately cover the dissection lesion, we placed two opened cell stents (Protégé, Medtronic, Minneapolis, MN, USA); one was an 8–6-mm tapered model with a length of 40 mm to cover the lesion from the distal CCA end to the proximal portion of ICA. Another was a 10-mm straight model with a length of 40 mm covering the CCA. Post-dilation was performed SterlingTM Balloon Dilation Catheter (Boston Scientific, Natick, MA, USA) with 5 × 20 mm covering both stents’ edges. The final angiography showed successful treatment with a smooth intra-arterial lumen (Fig. 4A). IVUS imaging showed sufficient stent coverage of the entire dissection lesion. It also confirmed a good expansion of the stent to the dissection area’s arterial wall with no plaque protruding the stents (Fig. 4B).
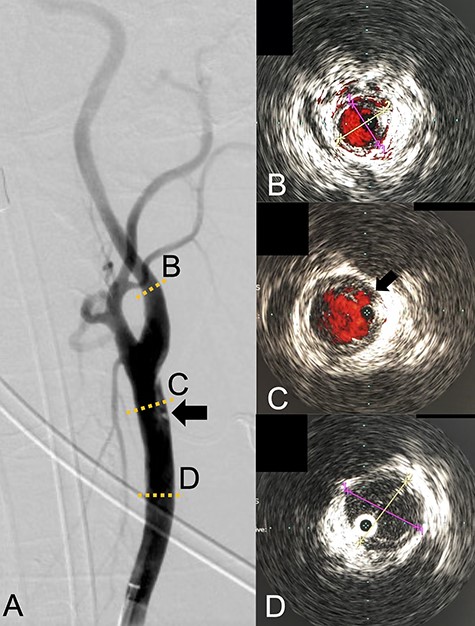
Angiography revealed the dissection starting proximal to the carotid bifurcation (Black arrow: A). IVUS provided the diameters of the ICA and CCA (B and D, respectively). IVUS imaging confirmed the pseudo-lumen of the dissection starting 1-cm proximal to carotid bifurcation (C) and the entry point of the dissection as well (Black arrow: C).
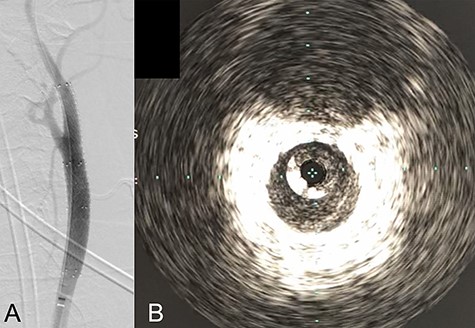
Angiography after stent placement showed favorable patency of the carotid artery with a smooth intra-arterial lumen (A). IVUS performed after stenting confirmed an excellent expansion of the stent to the dissection lesion’s arterial wall with no plaque protruding the stents (B).
DISCUSSION
Although CCA dissection is rare, it is nonetheless vital to rule out CCA dissection for all acute ischemic stroke patients, as carotid artery dissection is an independent causative factor for acute ischemic stroke [12]. There is no evidence-based treatment for CCA dissection. Conservative treatment includes antithrombotic and anticoagulation medications. On the other hand, surgical treatment comprises CAS, carotid endarterectomy and bypass surgery. The treatment goals are to: (i) prevent stroke recurrences, such as artery to artery embolism or progressive stenosis, and (ii) maintain adequate blood flow for distal cerebral perfusion regardless of the treatment selection [2]. Surgical treatment should be considered in case of high-risk stroke recurrence or inadequate cerebral perfusion. Only a few case reports for isolated spontaneous CCA dissection treated with CAS can be found so far [7, 8].
We would further like to note the usefulness of IVUS in this particular case. IVUS clearly visualized both the dissection’s entry point and the blood flow from true- to pseudo-lumen, the information of which is essential for treatment strategy planning. IVUS also provided helpful information to assess the stent’s coverage (Fig. 3). IVUS allowed us to predict the entire length of the arterial dissection before stent deployment [11]. Furthermore, IVUS provided important information for stent-size selection. Although the pseudo-lumen caused by the dissection made it difficult for us to precisely measure the true lumen diameter, IVUS allowed us to measure the diameter of the true lumen of the dissected artery, and we were able to select the appropriate stent for treatment. It also provided helpful information that proper stent apposition was established even after the stent deployment and post-balloon dilation, which indicated no additional angioplasty was necessary.
We have encountered a rare case of isolated spontaneous CCA dissection. To the best of our knowledge, this is the first case report of a CCA dissection treated with CAS assisted by IVUS. CAS assisted by IVUS may be an effective treatment option to prevent intraoperative complications and the recurrence of further stroke for isolated spontaneous CCA dissection.
ACKNOWLEGMENTS
The authors declare no financial supports that could be constructed as a potential conflict of interest.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
The study is funded by the Japanese government (Grant-in-Aid for Early-Career Scientists No.19K18374 from 2019 to 2021).



