-
PDF
- Split View
-
Views
-
Cite
Cite
Naoki Yanagawa, Masamichi Suzuki, Daisuke Saito, Ryo Sugimoto, Mitsumasa Osakabe, Noriyuki Uesugi, Kiyoto Shiga, Tamotsu Sugai, Coexistence of salivary duct, myoepithelial and epithelial-myoepithelial carcinomas in the parotid gland: a case report and literature review, Journal of Surgical Case Reports, Volume 2021, Issue 6, June 2021, rjab230, https://doi.org/10.1093/jscr/rjab230
Close - Share Icon Share
Abstract
Few reports have described two or more histologically-distinct carcinoma types within the same salivary gland. A 62-year-old man presented to our hospital after detecting a mass in the right parotid gland. Computed tomography revealed a tumor (5.1 × 5.0 cm) within the right parotid gland. Tumor resection with lymph node dissection was performed. The proliferation of three morphologically-different tumor cells was demonstrated on histopathologic examination (salivary duct carcinoma [SDC], myoepithelial carcinoma and epithelial-myoepithelial carcinoma [EMC]). The shape of the inner layer of cells in the EMC was similar to the SDC. Specifically, it appeared that the cells were a mixture of the two tumors with reciprocal transfer in the same area. Immunohistochemical staining showed that the SDC cells and the EMC inner cells were positive for AR, HER2 and p53. Thus, we suggest that our case represented a high-grade transformation.
INTRODUCTION
Few reports describe two or more histologically-distinct carcinoma types within the same salivary gland [1]. To date, these cases have been categorized as hybrid carcinomas, collision carcinomas or a high-grade transformation (HGT; [2–5]).
CASE REPORT
A 62-year-old man presented to our hospital after detecting a mass in the right parotid gland. He had no personal or family history of malignancy. On physical examination the mass was 5.0 × 5.0 cm in size with poor mobility. Computed tomography revealed a tumor (5.1 × 5.0 cm) within the right parotid gland that was enhanced by contrast (Fig. 1a). The tumor had an irregular shape with calcifications, a low-density area and the boundary between the tumor and surrounding tissues was indistinct. A malignant tumor of the right parotid gland was suspected, thus tumor resection with lymph node dissection was performed. The cut surface of the tumor was yellow–white with hemorrhage and necrosis (Fig. 1b). The tumor boundaries were ill-defined. The proliferation of three morphologically-different tumor cells was demonstrated on histopathologic examination. First, the cell type was atypical with abundant granular eosinophilic cytoplasm, large nuclei with coarse chromatin and prominent nucleoli. The cells were proliferative and invasive with duct-like, glandular and solid patterns (Fig. 2a and b). Frequent mitotic activity and necrosis were evident. The cells had the characteristics of a salivary duct carcinoma (SDC). Second, the atypical spindle-to-epithelioid cells with a clear cytoplasm were proliferative and invasive with trabecular, tubular and solid patterns (Fig. 2c and d). The stroma was hyalinized. The cells had the characteristics of a myoepithelial carcinoma (MC). Third, atypical duct-like structures were composed of two distinct cell layers. The inner layer was comprised of cuboidal cells with eosinophilic cytoplasm and round enlarged nuclei and the outer layer consisted of cells with clear cytoplasm and oval nuclei (Fig. 2e and f). The cells had the characteristics of an epithelial-myoepithelial carcinoma (EMC). In addition, the shape of the inner layer cell nuclei was similar to SDC. These three tumor types invaded the surrounding tissues and appeared to be a mixture of cells with reciprocal transfer in the same area (Fig. 3). Immunohistochemistry was performed using the Dako Envision+ System with dextran polymers conjugated to horseradish peroxidase (Dako, Glostrup, Denmark). The following primary antibodies (all purchased from Dako) were used: AR (AR441); αSMA (1A4); cytokeratin (AE1/AE3); EMA (E29); GCDFP-15 (23A3); polyclonal HER2; Ki-67 (MIB-1); p53 (DO7); p63 (DAK-p63); polyclonal S-100 and WT1 (6F-H2). The results of immunochemistry staining are shown in Table 1. Briefly, most of the SDC cells were positive for cytokeratin AE1/AE3, EMA, AR (Fig. 4a), HER2 (Fig. 4b) and p53. In contrast, most of the MC cells were positive for p63 (Fig. 4c), αSMA, S-100 (Fig. 4d) and WT-1, and weakly positive for cytokeratin AE1/AE3. Most of the EMC inner layer cells were positive for cytokeratin AE1/AE3 (Fig. 5a), EMA, and p53, and weakly positive for AR and HER2 (Fig. 5b). Most of the EMC outer layer cells were positive for p63, αSMA, S-100 and WT-1, and weakly positive for cytokeratin AE1/AE3 (Fig. 5a). In the mixed cell area, most of the EMC inner layer cells were positive for cytokeratin AE1/AE3, most of the EMC outer layer cells and MC cells were weakly positive for CK AE1/AE3 (Fig. 5c), and most of the EMC outer layer cells and MC cells were positive for p63 (Fig. 5d).
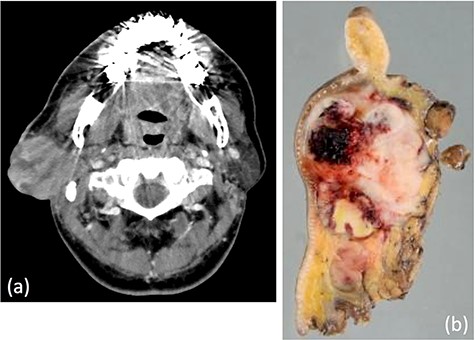
(a) Computed tomography revealed a tumor, 5.1 × 5.0 cm in size, within the right parotid gland enhanced by contrast. The tumor had an irregular shape with calcifications, low-density area and the boundary between the tumor and surrounding tissues was indistinct. (b) Macroscopically, the cut surface of the tumor was yellow–white with hemorrhage and necrosis. The tumor boundary was ill-defined.
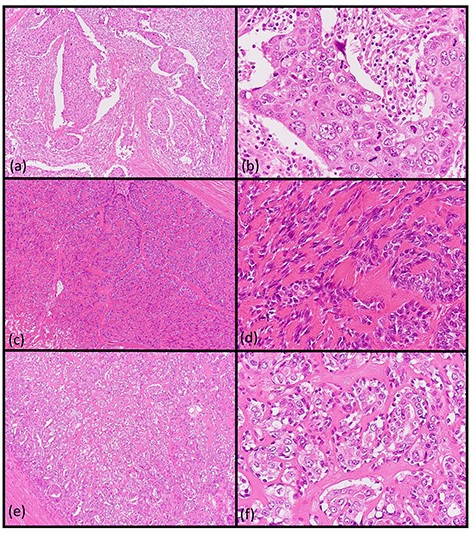
Histopathologic findings of the parotid tumor. (a and b: hematoxylin and eosin staining, ×40 and ×400) The cell type was atypical with abundant granular eosinophilic cytoplasm, large nuclei with coarse chromatin and prominent nucleoli. The cells were proliferative and invasive with duct-like, glandular and solid patterns. (c and d: hematoxylin and eosin staining, ×40 and ×400) The cell type was an atypical spindle-to-epithelioid cell with clear cytoplasm that was proliferative and invasive with trabecular, tubular and solid patterns. (e and f: hematoxylin and eosin staining, ×40 and ×400) The cell type was an atypical duct-like structure comprised of two distinct cell layers. The inner layer consisted of cuboidal cells with eosinophilic cytoplasm and round enlarged nuclei and the outer layer consisted of cells with clear cytoplasm and oval nuclei.
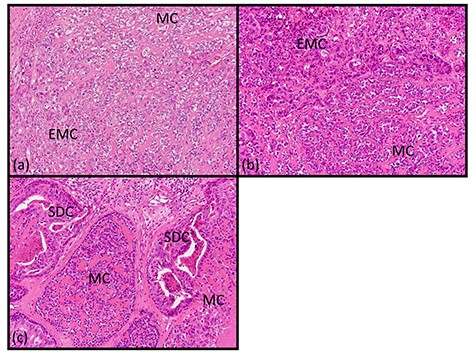
Histopathologic findings of transfer area. (a–c: hematoxylin and eosin staining, ×200) The tumor cells of the salivary duct, myoepithelial and EMC appeared to be a mixture with reciprocal transfer in the same area.
| . | . | CK AE1/AE3 . | EMA . | AR . | HER2 . | p63 . | SMA . | S-100 . | WT-1 . | p53 . | Ki-67 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDC | Posi | Posi | Posi | Posi | Nega | Nega | Nega | Nega | Posi | 50% | |
| MC | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 10% | |
| EMC | Inner cells | Posi | Posi | wPosi | wPosi | Nega | Nega | Nega | Nega | Posi | 40% |
| Outer cells | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 5% |
| . | . | CK AE1/AE3 . | EMA . | AR . | HER2 . | p63 . | SMA . | S-100 . | WT-1 . | p53 . | Ki-67 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDC | Posi | Posi | Posi | Posi | Nega | Nega | Nega | Nega | Posi | 50% | |
| MC | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 10% | |
| EMC | Inner cells | Posi | Posi | wPosi | wPosi | Nega | Nega | Nega | Nega | Posi | 40% |
| Outer cells | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 5% |
Abbreviations: MC, myoepithelial carcinoma; Nega, negative expression; Posi, positive expression; wPosi, weak positive expression.
| . | . | CK AE1/AE3 . | EMA . | AR . | HER2 . | p63 . | SMA . | S-100 . | WT-1 . | p53 . | Ki-67 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDC | Posi | Posi | Posi | Posi | Nega | Nega | Nega | Nega | Posi | 50% | |
| MC | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 10% | |
| EMC | Inner cells | Posi | Posi | wPosi | wPosi | Nega | Nega | Nega | Nega | Posi | 40% |
| Outer cells | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 5% |
| . | . | CK AE1/AE3 . | EMA . | AR . | HER2 . | p63 . | SMA . | S-100 . | WT-1 . | p53 . | Ki-67 . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SDC | Posi | Posi | Posi | Posi | Nega | Nega | Nega | Nega | Posi | 50% | |
| MC | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 10% | |
| EMC | Inner cells | Posi | Posi | wPosi | wPosi | Nega | Nega | Nega | Nega | Posi | 40% |
| Outer cells | wPosi | Nega | Nega | Nega | Posi | Posi | Posi | Posi | Nega | 5% |
Abbreviations: MC, myoepithelial carcinoma; Nega, negative expression; Posi, positive expression; wPosi, weak positive expression.
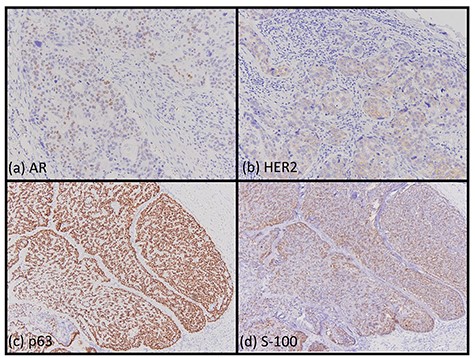
Immunohistochemical findings. Most of the cells of the SDC were positive for AR (a, ×200) and HER2 (b, ×200). Most of the cells of the myoepithelial carcinoma were positive for p63 (c, ×200) and S-100 (d, ×200).
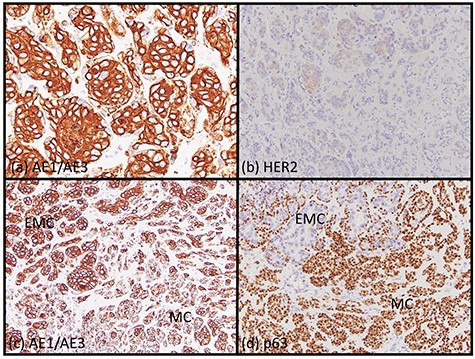
Immunohistochemical findings. Most of EMC inner layer cells were positive for cytokeratin AE1/AE3 (a, ×200) and weakly positive for HER2 (b, ×200). Most of the EMC outer layer cells were weakly positive for cytokeratin AE1/AE3 (a, ×200). In the mixed area, most of the EMC inner layer cells were positive for cytokeratin AE1/AE3 and most of the EMC outer layer cells and MC cells were weakly positive for CK AE1/AE3 (c, ×200). Most of the EMC inner layer cells were negative for p63, and most the EMC outer layer cells and MC cells were positive for p63 (d, ×200).
DISCUSSION
The presence of two or more histologically-distinct tumor entities in one topographic location is rare in salivary glands. When such cases are encountered, we need to consider differential diagnoses based on the potential relationship and pathogenesis of the tumor components, including hybrid tumors, collision tumors and malignant transformation or HGT [2–5]. First, we excluded collision carcinomas. We considered our case to be a hybrid carcinoma or HGT. Hybrid tumors are defined as ‘a neoplasm composed of two separate different tumor entities, each one of which conforms to an exactly defined tumor category, arising within the same topographical area. Both tumor entities are not separated but have an identical origin in the same topographical area’ [2]. We initially diagnosed this as a hybrid carcinoma; however, the precise criteria for a hybrid tumor/carcinoma have not been described in the WHO classification [6]. Moreover, the description, ‘hybrid tumors would not show evidence of evolution from one entity to another’ according to the diagnostic criteria of hybrid tumors, was deleted in the second edition of Gnepp’s Diagnostic Surgical Pathology of Head and Neck [7]. Hellquist et al. proposed criteria for a hybrid tumor/carcinoma that have not been universally agreed upon, and with the growing awareness of HGT the validity of the term, hybrid tumor, might be questioned [3]. In contrast, the concept of HGT in salivary gland neoplasms has been widely accepted, and the number of reported cases is rapidly increasing [3]. HGT in salivary neoplasms is most commonly associated with a poorly differentiated adenocarcinoma or undifferentiated carcinoma (hence the older term dedifferentiation). Dedifferentiation is defined as the abrupt transformation of low-grade (LG) well-differentiated carcinomas into a high-grade (HG) morphology, which lacks the original distinct histologic and immunohistochemical features [3, 8]. The LG and HG areas may be clearly demarcated, but a transitional zone is usually present [3]. We consider our case to represent a HGT (from EMC/MC to SDC) for the following reasons: three different carcinomas appeared as a mixture of cells with EMC inner layer cells exhibiting the same morphology and immunohistochemical staining as a SDC. Hamamoto et al. reported that their case had SDC and EMC components and inner ductal cells of double-layered EMC ducts with a similar morphology and immunophenotype to the SDC in the transitional area and suggested that a SDC originated from the EMC inner ductal cells [9]. We believe that our case was similar; however, the relationship between MC and EMC/SDC is unclear whether hybrid carcinoma or HGT.
In conclusion, we are of the opinion that our case was a HGT; however, the nomenclature of a hybrid tumor and HGT is ambiguous. Further examination, including next-generation sequencing, is warranted.
ACKNOWLEDGMENTS
The authors would like to thank the members of the Department of Molecular Diagnostic Pathology, Iwate Medical University for their support.
FUNDING
The author(s) received no financial support for the research, authorship and/or publication of this article.
CONFLICT OF INTEREST STATEMENT
The author(s) declare no potential conflicts of interest with respect to the research, authorship, or publication of this article.
INFORMED CONSENT
Written informed consent was obtained from the patient’s legal authorized representatives for publication of this case report and accompanying images.



