-
PDF
- Split View
-
Views
-
Cite
Cite
María J Irarrázaval-Mainguyague, Manuel Cabreras, Sebastián Oksenberg, María A Pulgar, Francisco Rojas, Manuel Álvarez, Felipe F Quezada-Diaz, Malakoplakia mimicking a locally advanced colorectal neoplasm, Journal of Surgical Case Reports, Volume 2021, Issue 6, June 2021, rjab225, https://doi.org/10.1093/jscr/rjab225
Close - Share Icon Share
Abstract
Malakoplakia is a rare disease. Clinical presentation is non-specific, making its diagnosis an incidental finding on histopathological analysis. The aim of this case report is to describe a patient diagnosed with colon, renal and soft tissue malacoplakia mimicking a locally advanced colorectal cancer.
A 75-year-old man was admitted due to intense abdominal pain. No relevant findings at the physical examination. Computed tomography showed parietal thickening of the descending colon with left kidney, iliopsoas muscle and retroperitoneum involvement. An elevated blood serum creatinine, elevated glycated hemoglobin and urinary infection were detected. Surgery was decided for suspicious symptomatic colonic neoplasm. Left segmental colectomy with left partial nephrectomy and retroperitoneal soft tissue resection was performed. Pathology report was compatible with malakoplakia.
Malakoplakia is a rare disease and may affect multiple organs. Because there are no clinical-specific findings, diagnosis is usually made with histopathological study of the surgical specimen.
INTRODUCTION
Malacoplakia is a rare granulomatous inflammatory disease, with less than 500 new cases reported per year in the USA [1].
First described in 1902 by Michaelis and Gutman [1], it affects more frequently the urinary tract and gastrointestinal system, specially the descending colon, sigmoid and rectum [2].
Clinical presentation is non-specific and depends on the type of organ affected, including symptoms such as diarrhea, abdominal pain, gastrointestinal bleeding and/or bowel obstruction [3, 4].
The diagnosis can be challenging and most frequently correspond to an unexpected pathological finding.
Here, we describe a case of colon, renal and soft tissue malakoplakia mimicking an advanced colorectal neoplasm.
CASE REPORT
This is a 75-year-old man with a past medical history of diabetes mellitus and hypertension. He had a history of 6 months of an intense left flank pain. Physical exam was unremarkable. An abdominal ultrasound described a left renal mass. Computed tomography (CT) showed a 7.3 × 5 cm mass in the descending colon infiltrating the left kidney, iliopsoas muscle and retroperitoneum. No distant metastases were observed (Fig. 1).
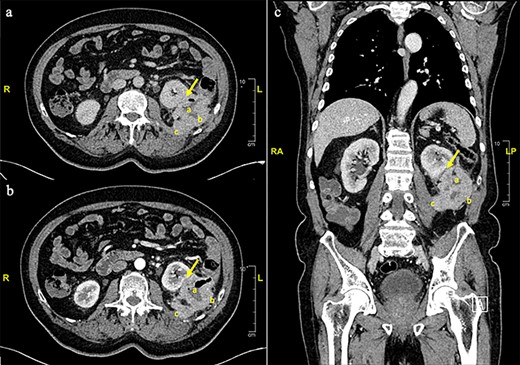
Contrast enhanced thorax, abdomen and pelvis computed tomography scan. a. Axial plane, non-contrast phase. s. Axial plane, venous phase. c. Coronal plane. An eccentric parietal thickening, sized 7.3 × 5 cm. With a cavitated center was observed (a), anteriorly displacing the left kidney with no clear cleavage plane in its inferior third (arrow). A loss of cleavage plane with the posterior-lateral left abdominal wall (b) and the psoas-iliac muscle (c) was also described.
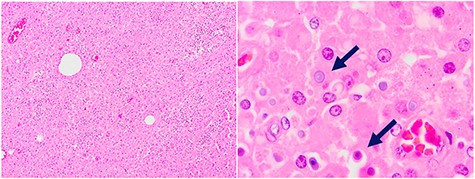
Biopsy: extensive chronic inflammatory reaction with macrophages and Michaelis–Gutmann bodies (arrows).
Laboratory tests showed increased serum creatinine (2,0 mg/dl) and glycosylated hemoglobin (8,8%) levels. Carcinoembryonic antigen was normal (2.8 ng/ml). A urine culture was positive for Escherichia coli.
Surgery was decided due to high suspicion of a symptomatic locally advanced colorectal neoplasm. Intraoperatively, a tumoral mass was founded, involving the descending colon, left kidney, lateral abdominal wall and retroperitoneum. A left segmental colectomy with primary colonic anastomosis, left partial nephrectomy and retroperitoneum soft tissue resection was performed. Postoperative recovery was uneventful. Patient was discharged on postoperatory Day 6.
Pathology described a big mass of 8.5 × 5.3 × 3.5 cm infiltrating the pericolic adipose tissue and the colonic mucosa. Microscopically, an extensive chronic inflammatory reaction was observed involving the colonic wall, renal tissue and muscle, corresponding mainly to macrophages and Michael–Gutman bodies (Fig. 2), compatible with malakoplakia.
During follow-up, patient reported resolution of symptoms and no major deterioration in renal function was observed.
DISCUSSION
Malakoplakia is an uncommon granulomatous inflammatory disease. It affects more frequently patients with chronic conditions such as diabetes mellitus, human immunodeficiency virus, neoplasms, organ-transplantation and immunosuppressive therapy [1, 3, 5]. Current knowledge about this condition is based on case reports and small series. Zhang et al. [2] described one of the larges published series including 26 patients with gastrointestinal malakoplakia, 88% of the cases affecting colon and rectum. The median age of presentation was 64 years, with a higher rate of women (62%). Diagnosis was an incidental finding in 63% of the cases, generally in the context of colorectal cancer screening [2]. Interestingly, colorectal cancer was simultaneously diagnosed in 19% of the patients. This association between adenocarcinoma and malakoplakia has been previously described [2, 6].
Clinical diagnosis is uncommon. Differential diagnosis of malakoplakia is broad, including conditions like solid neoplasms, inflammatory bowel disease, xanthogranulomatous pyelonephritis, tuberculosis and Whipple disease [1, 2, 5, 7, 8]. Radiological findings are also highly unspecific [4, 9]. Multifocal lesions are presented up to 75% of the cases, sometimes converging in a big unique mass, simulating an infiltrative neoplasm [9, 10]. In our case, CT images evidenced a big mass in relation to the left colon. The final diagnosis is frequently made based on the pathology analysis of the surgical specimen [1, 5].
Macroscopically, malakoplakia lesions may follow three patterns: single yellow mucosal plaques, multinodular or polypoidal lesions and/or large mass lesions [1, 3, 5]. Microscopically, the presence of Michaelis–Gutman bodies is pathognomonic for malakoplakia but not essential, since they can be absent in early phases of the disease [1, 8, 11, 12]. Michaelis–Gutman bodies are granular basophilic structures and can be observed inside macrophages or, extracellularly, among collagen-produce fibroblasts [1].
The etiology of malakoplakia is not well understood. Possible pathogenic mechanisms included infections due to Gram-negative microorganisms, particularly E. coli, and abnormal immune response secondary to defective lysosomal function in macrophages [1, 6, 8, 10, 11]. The accumulation of partially digested microorganisms in macrophages due to microtubular and lysosomal dysfunction is accepted as the most common disease mechanism [1, 8]. In our patient, a possible immunosuppressive condition secondary to the long-term diabetes mellitus and a urinary tract infection may have contributed to malakoplakia development. This sequence of events has been previously described [8].
Even though clinical diagnosis is challenging, it may avoid unnecessary surgical interventions. It is known that malakoplakia may respond adequately to medical treatment [5, 8]. There is currently no therapy considered gold standard for malakoplakia. Among described alternatives are the suspension of immunosuppressive therapy, control of the underlying immunosuppressive condition, cholinergic agents, and long course treatment with antibiotics that affect directly macrophages (i.e. quinolones, trimethoprim-sulfamethoxazole) [3, 5, 13]. Surgical resection is indicated in cases of extensive organ infiltration, marked symptoms and/or suspected malignancy. Endoscopic resections have been described in cases of localized lesions [7, 13].
In conclusion, malakoplakia is an uncommon disease and can compromise any organ, with the gastrointestinal tract being the second most frequent site involved. It generally affects patients with immunosuppressive conditions or chronic diseases. Even though the clinical presentation is non-specific and is generally diagnosed postoperatively, a preoperative diagnosis may avoid an unnecessary surgical intervention.
References
- abdominal pain
- computed tomography
- colorectal cancer
- physical examination
- urinary tract infections
- hemoglobin
- colonic neoplasms
- colorectal neoplasms
- malacoplakia
- retroperitoneal space
- surgical procedures, operative
- colon
- diagnosis
- kidney
- pathology
- colon resection, partial
- partial nephrectomy
- iliopsoas muscle
- descending colon
- kidney, left
- serum
- creatinine increased
- soft tissue resection
- histopathology tests
- incidental findings
- rare diseases
- soft tissue
- surgical specimen



