-
PDF
- Split View
-
Views
-
Cite
Cite
Ved Prakash R Cheruvu, Manal M Khan, Solitary cysticercus in the right temporalis muscle: case report of a rare form of presentation of cysticercosis, Journal of Surgical Case Reports, Volume 2021, Issue 6, June 2021, rjab223, https://doi.org/10.1093/jscr/rjab223
Close - Share Icon Share
Abstract
Solitary cysticercus localized to the head and neck musculature is an unusual form of presentation of cysticercosis. Since it is rare and has non-specific manifestations, it can present a diagnostic challenge to the clinician. Our patient was a 16-year-old female who presented with a gradually increasing, painful swelling over right temple region of 6-month duration. Ultrasound and computed tomography scan revealed the presence of a solitary cysticercus in the right temporalis muscle. Surgical excision of the lesion was combined with a 4-week course of the anti-helminthic drug, Albendazole. Patient had a satisfactory resolution of symptoms and there was no recurrence in a follow-up period of 3 years. We suggest that cysticercosis should be considered as one of the possibilities in the differential diagnosis of swellings in the maxillofacial region, especially in the endemic areas. Imaging studies play an important role in confirmation of the diagnosis.
INTRODUCTION
Cysticercosis is a parasitic affliction of the tissues caused by Cysticercus cellulosae, which is the larval stage of pork tape worm Taenia solium. It was described for the first time by Johannes Udalric Rumler in 1555 [1]. It commonly occurs in the areas where there is a combination of uncontrolled pig breeding, poor sanitation and close interaction between humans and animals. This condition is endemic in the developing countries of Asia, Africa and Latin America. In 2010, cysticercosis was designated as one of the 17 ‘neglected tropical diseases (NTDs)’ of worldwide public health importance by the World Health Organization (WHO). In the year 2015, the WHO’s Foodborne Disease Burden Epidemiology Reference Group established that T. solium was a leading cause of mortality from food-borne illnesses, resulting in 2.8 million disability-adjusted life-years [2].
Cysticercus cellulosae is found in muscles and other tissues of pigs that usually serve as intermediate hosts, whereas humans are the definitive hosts and harbor the adult worm. Pigs acquire the infection when they ingest contaminated food or water that contains proglottids or eggs from human feces. The eggs develop into cysticerci in pig muscles.
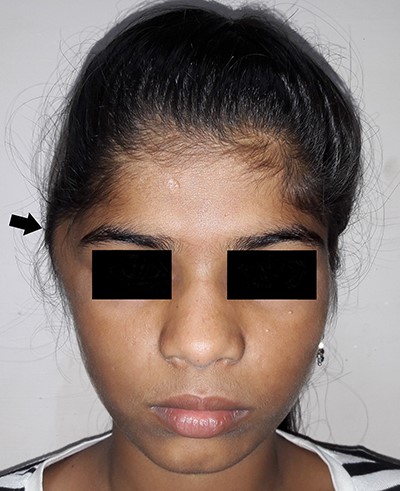
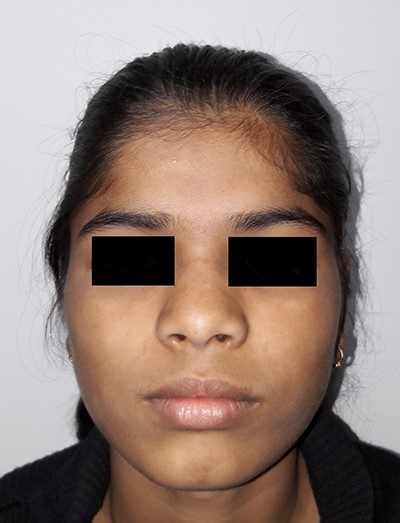
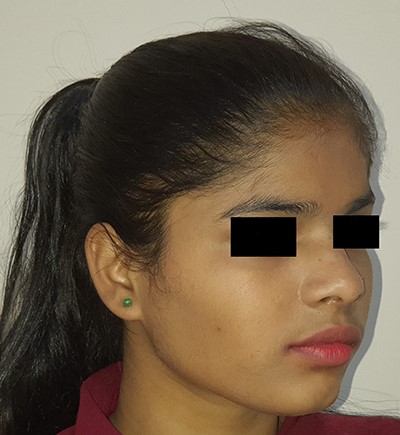
Picture at initial presentation, frontal view, arrow indicates the swelling.
Human infection occurs when these are ingested through the consumption of raw or undercooked pork. Humans can also acquire infection through T. solium eggs due to poor hygiene (via fecal–oral route) or ingestion of contaminated water or food [2]. In this case, humans become the accidental intermediate hosts. These ova are digested in the stomach and oncospheres are released, which penetrate the intestinal wall and reach the circulation [3]. This may lead to cysticercosis that commonly involves the brain, meninges and eyes, which together constitute 86% of the cases. Less commonly they are located in the muscles, subcutaneous tissues, liver, heart, lungs and peritoneum [4]. Rarely, they are localized to the oral and perioral tissues like the muscles of mastication, muscles of facial expression, suprahyoid muscles, post-cervical musculature, tongue, buccal mucosa and lips [5–7]. Isolated head and neck muscular cysticercosis without the involvement of central nervous system is rare and only few cases are reported in the literature [8]. These cases have non-specific clinical manifestations and present a diagnostic challenge to the clinician [9].
CASE REPORT
A 16-year-old female patient had presented to us with the complaint of a gradually increasing painful swelling over the right temple region of 6 months duration. She had a history of minor trauma to the site due to a fall 6 months back. There was no restriction in mouth opening or difficulty in chewing. There were no symptoms suggestive of neurological involvement.
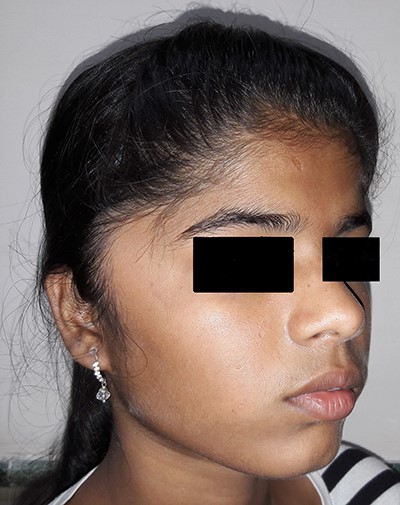
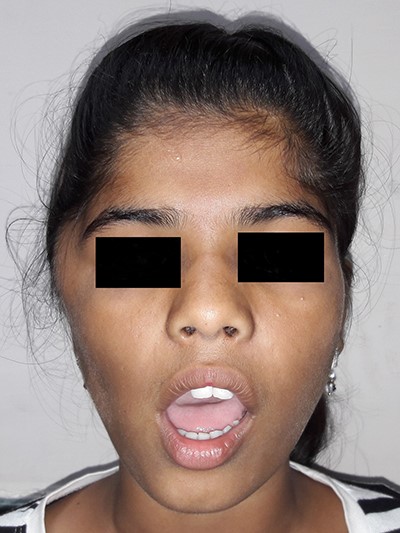
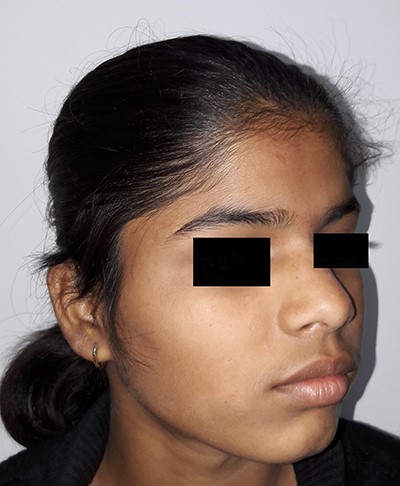
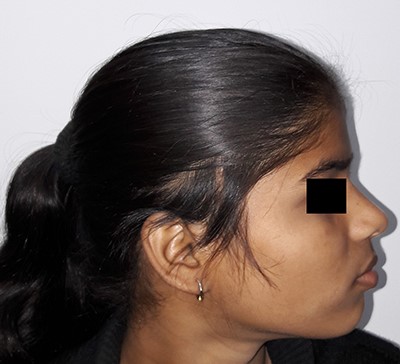
On examination, there was a solitary swelling in the right temporal region with ill-defined margins. It had a soft to firm consistency and was mildly tender on palpation. Mouth opening was adequate (Figs 1–3). There were no other significant findings on examination.
Ultrasound revealed a hypoechoic cystic lesion in the right temporalis muscle with an eccentric hyperechoic nodule. Computed tomography (CT) scan confirmed the presence of a well-defined cystic lesion with an eccentric hypointense nidus in the right temporalis muscle. There were no other similar lesions in the head and neck region. These features suggested a diagnosis of a solitary cysticercosis involving the right temporalis muscle.
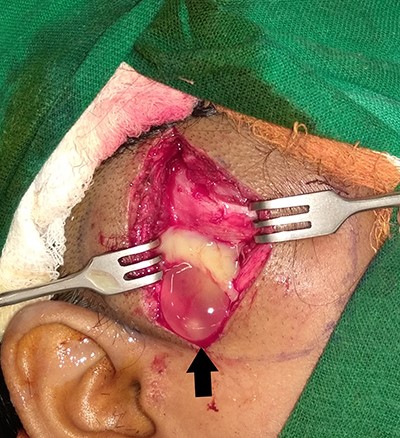
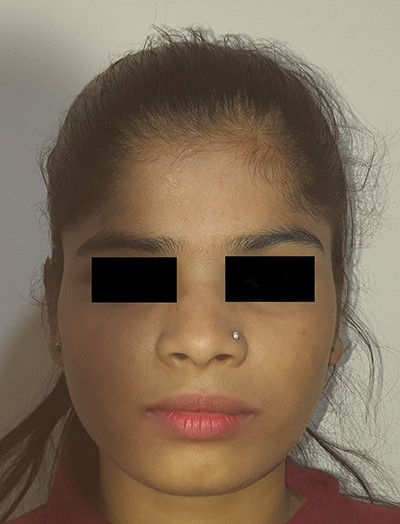
Patient was initially kept on conservative treatment with Albendazole 15 mg/kg body weight/day in two divided doses for 2 weeks. As there was no relief in the pain or swelling, we decided to go for surgical exploration. The lesion was approached through a vertical incision in the right temporal region. Intraoperatively, ~5 ml of pus and a white opalescent cystic lesion, within a cavity were found (Fig. 4). The cavity and surgical field were thoroughly irrigated with Povidone-Iodine before closing the incision over a suction drain. Culture of the pus did not yield growth of any microbes. The suture line healed well and the swelling resolved (Figs 5–7). Histopathological examination confirmed the lesion to be a cysticercus cyst.
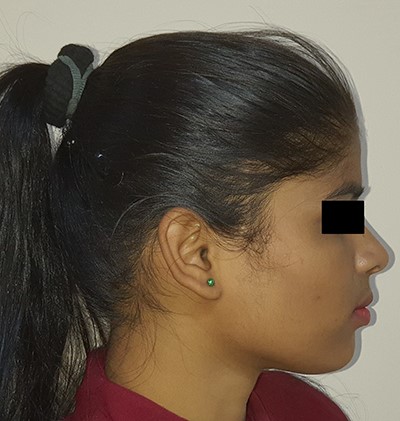
Albendazole was continued for 2 more weeks. Patient was followed-up regularly and there was no recurrence during a 3-year follow-up period (Figs 8–10).
DISCUSSION
The clinical course of cysticercosis depends on the number of lesions, the tissues affected and the reaction of the tissues to these organisms [6]. Commonly, patients present with multiple lesions such as cysts of different sizes or multiple nodular calcifications and these may be associated with varying degrees of host response [10]. Cysticercosis may remain asymptomatic for a variable period or present with severe local symptoms. Various types of clinical presentations such as myalgic, myopathic, nodular or mass like and the rare pseudohypertrophy have been reported [9]. The cysts are of ovoid or round shape, white or opalescent color, up to 1.5-cm size and contain an invaginated scolex with hooklets [9].
Isolated head and neck muscle cysticercosis is unusual and solitary cysticercosis localized to temporalis muscle is rarer [11]. Since it is rare and has non-specific manifestations, it can present a diagnostic challenge to the clinician [9].
Imaging studies (ultrasonography, CT or magnetic resonance imaging) play an important role in the diagnosis of cysticercosis. Vijayaraghavan described four kinds of sonographic appearances of muscular cysticercosis [12].
Treatment of cysticercosis should be based on the clinical manifestations and the tissues affected. Praziquantel and Albendazole are the recommended anti-helminthic medications [13]. Surgical excision is the treatment of choice for ventricular, ocular, spinal and symptomatic subcutaneous cysticerci [14].
In the maxillofacial region, prognosis is favorable in contrast to the seriousness of the disease in the cerebral, ocular and cardiac sites [15]. There are no reported recurrences after treatment.
We suggest that cysticercosis should be considered as one of the possibilities in the differential diagnosis of swellings in the maxillofacial region, especially in the endemic areas. Imaging studies play an important role in confirming the diagnosis and should be sought out in cases with non-specific clinical manifestations.
ACKNOWLEDGMENTS
We acknowledge the contributions of our residents and staff in helping with the patient care and follow-up.



