-
PDF
- Split View
-
Views
-
Cite
Cite
Karishma Kodia, Stephanie Richards, Andre Pinto, Joseph M Pearson, Dao M Nguyen, A 20-year latency between hysterectomy for endometrial adenocarcinoma and solitary pulmonary metastasis: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 2, February 2021, rjaa595, https://doi.org/10.1093/jscr/rjaa595
Close - Share Icon Share
Abstract
We present a rare case pulmonary metastasis of an early stage endometrial cancer nearly 20 years after curative surgical resection. Our patient had a remote history of hysterectomy for endometrial cancer in 1998 and later had Stage 1B right upper lobe lung cancer treated with lobectomy and adjuvant chemotherapy in 2014. She was found to have an enlarging nodule in the left upper lobe in 2018, which was thought to be another primary lung cancer. She underwent left upper lobe segmentectomy for an intraoperative diagnosis of adenocarcinoma by diagnostic wedge resection of the lung nodule. Final pathologic examination of the resected tumor demonstrated an endometrial adenocarcinoma. It is important for thoracic surgeons to remain vigilant, keeping secondary lung cancer in the differential diagnosis for patients with complex oncologic histories.
INTRODUCTION
Remote solitary pulmonary metastases from primary endometrial carcinomas are rare, with the incidence of isolated pulmonary metastasis between 2.3 and 7%; 80% of these metastases occur within 3 years of endometrial surgical management [1]. Following curative resection of endometrial adenocarcinoma, the longest reported period for a local vaginal recurrence is 26.5 years and 17 years for an isolated pulmonary metastasis [2]. We report a case of an isolated lung metastasis, found incidentally, from an endometrial carcinoma treated by hysterectomy almost 20 years prior. The lesion was detected as a left upper lobe lung nodule by semi-annual chest computed tomography (CT) scan for routine surveillance following a recent curative resection of a right upper lobe (RUL) primary lung adenocarcinoma.
CASE PRESENTATION
A 77–year-old female presented to the thoracic surgery clinic in April 2014 with a right lung mass. She was previously healthy and experienced chest pain, prompting imaging studies revealing a RUL lung mass. Her medical history was significant for an endometrial adenocarcinoma in 1998 treated with total abdominal hysterectomy and a remote 20 pack-year history of cigarette smoking. Chest CT scan showed a 3.6 × 2.9 cm RUL mass, strongly fluorodeoxyglucose (FDG) avid by positron emitted tomography (PET) scan. Pre-resection biopsy revealed a primary lung adenocarcinoma. She underwent robotic thoracoscopy, right upper lobectomy, and complete mediastinal lymph node dissection. Pathologic examination revealed a 5.5 cm moderately differentiated acinar-predominant TTF-1- and Napsin A-positive primary lung adenocarcinoma and multiple foci of atypical adenomatous hyperplasia (AAH) in the background of lung parenchyma (Fig. 1) without evidence of intrathoracic lymph node metastasis (N0). She had an unremarkable post-operative course and received four cycles adjuvant chemotherapy for Stage 1B primary lung cancer. Semi-annual surveillance CT scans showed no evidence of loco-regional recurrence of the RUL lung cancer until a chest CT scan in May 2018, 4 years after the right upper lung lobectomy, demonstrated a 1.2 cm left upper lobe lung nodule that was FDG-avid on the follow-up PET scan. Review of previous chest CT scans revealed this lesion was present in 2014 and had not shown signs of progression until 2018. This lesion was thought to be another primary lung cancer, given her recent history of primary lung cancer and very remote history of endometrial adenocarcinoma. She underwent a left robotic thoracoscopy and diagnostic wedge resection for tissue diagnosis by intraoperative frozen-section pathologic examination, which showed adenocarcinoma. A lingular segmentectomy and intrathoracic lymphadenectomy was then performed for presumed primary lung adenocarcinoma. Final pathologic examination of the resected left lung cancer revealed a PAX-8 and ER-positive low-grade adenocarcinoma, negative for TTF-1 and Napsin A, consistent with metastatic endometrioid adenocarcinoma (Fig. 2) and no lymph node metastases. After multidisciplinary tumor board discussion, the patient was started on hormonal therapy with letrozole. She has been doing very well since her second lung resection, and recent surveillance chest CT scan in November 2019 does not show evidence of tumor recurrence.
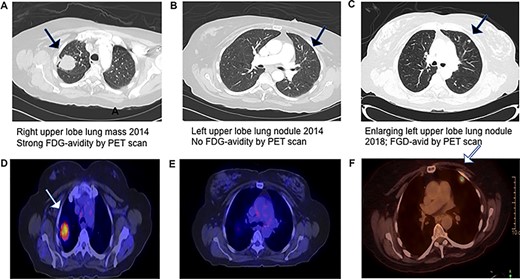
Progression of CT and PET scans between 2014 and 2018, which demonstrates that the left upper lobe nodule initially had no FDG-advidity but subsequently had an interval increase in size and SUV of 7.1.
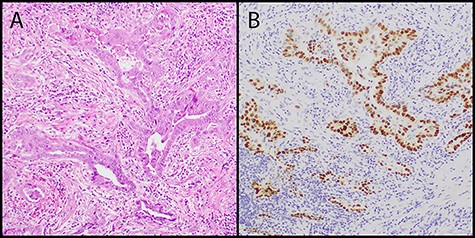
(A) low power image showed adenocarcinoma with angulated acinar architecture in a background of desmoplastic stroma (hematoxylin and eosin stain, original magnification 100×); (B) the tumor cells showed positive nuclear staining for TTF-1 consistent with lung origin (TTF-1 immunostain, original magnification 100×).
DISCUSSION
Endometrial carcinoma is the most common gynecologic malignancy in the USA, with recurrence roughly 13% [3, 4]. Pulmonary metastases are the most common site of extra-pelvic metastasis [5]. Isolated pulmonary recurrences occur in 2.3–7% of cases, with 80% metachronous and 20% synchronous lesions [3, 6]. Treatment includes total hysterectomy with bilateral salpingo-oophorectomy. An important prognostic marker of subsequent metastatic disease is invasion of the outer third of the myometrium, portending a 5-fold increase in lymph node involvement and distant metastasis [7]. Factors associated with improved survival of pulmonary metastasis include: disease-free interval over 12 months, Grade 1–2 histology, estrogen receptor (ER) positivity, <50% myometrial invasion, primary lesion <2 cm in size, unilateral lesions and <5 nodular lesions of the lung [8]. The longest latency of locoregional (vaginal vault) recurrence is 26.5 years. Falkenstern et al. [2] reported that the longest intervals between the initial gynecological surgery to pulmonary metastatic diagnosis in two cases were 14 and 17 years. Similarly, Ito et al. [6] reported an isolated pulmonary metastasis 17 years after initial endometrial cancer diagnosis.
We present a case of a single isolated lung metastatic lesion appearing almost 20 years after primary endometrial adenocarcinoma resection. The pre-operative diagnosis of a pulmonary metastasis was confounded by the recently treated primary RUL adenocarcinoma and the very remote history of early stage endometrial adenocarcinoma. A pre-resection biopsy for tissue diagnosis and immuno-histochemical staining for tumor markers may have revealed the tissue origin of the tumor and dictated the type of pulmonary resection. Therapeutic wedge resection with normal lung margin of at least half the tumor size, and not necessarily an anatomic resection, is indicated for pulmonary mastectomy with intrathoracic lymph node systemic sampling [9]. The left upper lobe tumor was small and in a subpleural location amenable to intraoperative wedge resection for tissue diagnosis; as such, we could bypass transthoracic needle biopsy and its inherent complications to provide immediate therapeutic intervention—in this case, an anatomic segmentectomy for a presumed second primary lung cancer [10]. One can argue that a segmentectomy for a small, peripherally located pulmonary metastasis is an over-treatment; however, a non-anatomic therapeutic wedge resection is a gross under-treatment of a primary lung cancer.
In summary, to the best of our knowledge, this case represents the longest interval of nearly 20 years between hysterectomy for endometrial cancer and clinical presentation of a single lung metastasis. Recent RUL lung cancer, the remote nature of her endometrial cancer and radiographic presentation of a single lung nodule confounded our ability to consider a lung metastasis as part of the differential diagnosis.
CONFLICT OF INTEREST
None declared.
References
- adenocarcinoma
- adjuvant chemotherapy
- endometrial cancer
- differential diagnosis
- hysterectomy
- intraoperative care
- mastectomy, segmental
- wakefulness
- diagnosis
- thoracic surgery specialty
- lung cancer
- metastasis to the lung
- pulmonary nodule
- lobectomy
- wedge resection
- endometrial adenocarcinoma
- excision
- tumor excision



