-
PDF
- Split View
-
Views
-
Cite
Cite
Jennifer Walsh, Neil Fennelly, Clive Kilgallen, Eabhann O Connor, James Forde, Binu Dinesh, Fidelma Fitzpatrick, Expect the unexpected: chronic renal abscess secondary to renal actinomycosis, Journal of Surgical Case Reports, Volume 2021, Issue 12, December 2021, rjab536, https://doi.org/10.1093/jscr/rjab536
Close - Share Icon Share
Abstract
Actinomycosis is an invasive infection, which can affect numerous anatomical sites, though rarely the kidney. The rate of nephrectomy is high despite antibiotic therapy.
A 51 year old presented with a Proteus mirabilis renal abscess 9 years following a similar renal abscess. The abscess persisted despite appropriate antibiotic treatment and radiological drainage. In addition to P. mirabilis, Actinomyces species was isolated on polymicrobial abscess culture after 6 weeks antibiotic therapy. Despite appropriate antibiotics, nephrectomy was required. Histology confirmed actinomycosis. Actinomycosis should be considered in chronic, destructive infections, especially if failure to respond to appropriate antimicrobials. However, Actinomyces species may be missed by routine culture techniques. Because of the polymicrobial nature of abscesses, good communication with the laboratory is essential to ensure that cultures are prolonged and the isolation of one pathogen does not hinder the isolation of others.
CASE REPORT
A 51-year-old female presented with a 4-day history of fevers and left flank pain. Nine years earlier, she was treated for a Proteus mirabilis renal abscess. She was treated empirically with intravenous (IV) cefuroxime and gentamicin. A 1.0 cm left renal calculus and a 7.2 × 5.3 × 5.0 cm heterogeneous, mostly cystic lesion with some internal vascularity at the interpolar region of the left kidney was found on renal ultrasound (US), suggestive of a renal abscess. US-guided aspirate grew P. mirabilis, resistant to amoxicillin and susceptible to co-amoxiclav, cefuroxime and gentamicin. Blood cultures were sterile. A left-sided ureteric stent was inserted for management of a presumed obstructing stone. She was treated with IV cefuroxime for 14 days followed by oral co-amoxiclav for 4 weeks.
Five months later, 1 week after elective lithotripsy, she was readmitted with left flank pain, rigours and hypotension. P. mirabilis resistant to co-amoxiclav and susceptible to cefuroxime was cultured from blood and urine. Computed tomography (CT) abdomen demonstrated a 6.8 × 4.8 cm abscess within the upper pole of the left kidney and an 8 mm stone medial to the collection. She was treated with IV cefuroxime, however remained unwell and febrile. A repeat CT demonstrated interval development of 8.6 × 5.8 × 10.9 cm subcapsular gas and fluid collection in the posterior left kidney. There was extracapsular extension. There appeared to be a small anterior communication between this subcapsular collection and the chronic anterior left mid pole collection, which demonstrated new locules of gas.
A drain was inserted and purulent fluid aspirated. This grew a light growth of P. mirabilis resistant to co-amoxiclav and susceptible to cefuroxime and ceftriaxone following 1 day of incubation, a heavy growth of Actinomyces radingae following 2 days incubation, and a heavy growth of an anaerobe, susceptible to metronidazole on Day 6. In view of the chronic nature of the abscess and heavy growth of Actinomyces IV cefuroxime was changed to IV ceftriaxone. Metronidazole was added for anaerobic cover. Repeat CT 1 week later indicated ongoing infection. Further drain fluid specimens grew a moderate growth of P. mirabilis as above at Day 1, mixed anaerobes susceptible to metronidazole and a moderate growth of Actinomyces urogenitalis at Day 4. Both A. radingae and A. urogenitalis were resistant to clindamycin and susceptible to co-amoxiclav, piperacillin-tazobactam, ceftriaxone, meropenem and vancomycin.
The ureteric stent was removed as urine reflux with a possible ruptured calyx were thought to be potential factors that could lead to chronic infection. Follow-up CT 1 week later showed increase in the chronic collection within the anterior aspect of the kidney. The drain was within the posterior collection which had decreased in volume.
As the patient was stable she was discharged via Outpatient Antibiotic Therapy services on IV ceftriaxone and oral metronidazole. After discharge, she described a poor quality of life and constant pus from the drain.
She was admitted emergently following a collapse when the drain fell out. Unchanged renal abscesses were seen on CT with extensive multiloculated abscesses along the line of the previous percutaneous pigtail catheter extending out within the soft tissues of the left flank. A drain was reinserted and the patient improved clinically. Renogram demonstrated only 20% renal function. Twelve days later a laparoscopic nephrectomy was performed.
Macroscopic examination demonstrated extensive replacement of renal parenchyma with fatty and cystic purulent material. Numerous necrotic appearing nodules were seen (Fig. 1). Histology revealed multiple cavitating abscesses, chronic inflammation and sclerotic tissue. The macroscopically identified nodules histologically comprised of rod-shaped branching organisms, typical of Actinomyces species, with adjacent pink–purple granules consistent with sulphur granules (Fig. 2). The nature of these organisms were confirmed with Grocott methanimine silver and Gram stains. Microbiology testing was not performed as the sample had been placed in formalin. Post-operatively antibiotics were stopped and she was discharged home after 4 days.
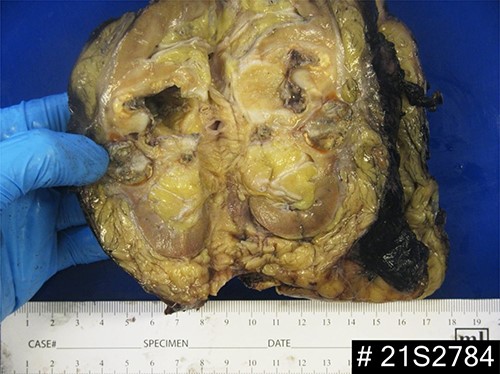
Macroscopic appearance of the left kidney demonstrating necrotic nodules, dilation of the pelvicalyceal system and extensive replacement of the renal parenchyma with fatty and cystic purulent material.
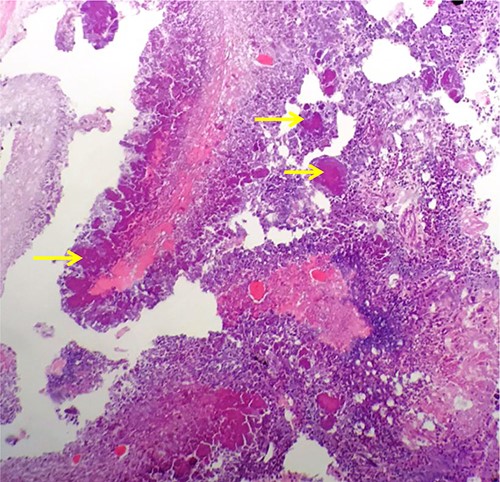
Haematoxylin and Eosin stained formalin-fixed paraffin embedded kidney showing rod-shaped branching microorganisms typical of Actinomyces, with adjacent purple–pink granules consistent with sulphur granules (arrows).
Actinomyces species are non-spore-forming, filamentous Gram-positive anaerobic or microaerophilic bacteria that colonize the oropharynx, gastrointestinal and urogenital tract. Invasive infection (actinomycosis) though rare can affect a number of anatomical sites including the mandible, breast, gastrointestinal and genitourinary tract. Actinomyces israelii is the most commonly reported species in clinical cases [1]. Renal actinomycosis is rare and <25 cases have been reported since 1990 in adult patients [2]. It may present as a renal abscess, pyonephrosis with renal calcinosis or necrotizing papillitis. Most cases have been described in immunocompetent individuals [3]. Treatment is usually high dose IV penicillin for 6 weeks followed by oral penicillin for 6–12 months. In cases of renal actinomycosis, the rate of nephrectomy remains high despite antibiotic therapy [4].
Isolation and identification of Actinomyces species can be challenging and may be inhibited by previous antibiotic therapy, inappropriate transport of specimens, inadequate culture conditions and inadequate incubation times. Actinomyces species may be missed by routine culture techniques and incubation periods, as they can be fastidious and slow-growing, growing best under anaerobic conditions. Direct Gram-staining is essential and has been shown to have higher sensitivity compared to culture [1]. All pus samples received in the microbiology laboratory are routinely cultured onto media which facilitates the growth of common organisms such as Staphylococci, Streptococci and Enterobacterales and incubated for 48 hours minimum. In order to detect Actinomyces species specimens must be inoculated on blood agar supplemented with metronidazole and nalidixic acid and incubated anaerobically for 10 days [5]. This may not be routinely performed in microbiology laboratories without communication of clinical details. The initial specimens in this patient grew P. mirabilis only. P. mirabilis commonly displays zonal growth (‘swarming’) which interferes with growth of other microorganisms. It was only after antibiotic therapy, which likely reduced the amount of P. mirabilis in the abscess, that Actinomyces species were cultured. In addition, as the clinical microbiology team had been contacted in advance, the laboratory had a high index to search for other pathogens. Otherwise, in the case of a renal abscess, though many infections can be polymicrobial, the isolation of Gram-negative bacteria such as P. mirabilis are felt to represent the primary pathogen. As a result, further culture conditions to facilitate the growth of Actinomyces may not be performed. Lynch et al. highlighted that Actinomyces species are frequently unidentified or misidentified using matrix-assisted laser desorption ionization-time of flight mass spectrometry. The current gold standard for species-level identification is 16S rRNA sequencing [6].
This case highlights the need to consider actinomycosis in any patient with a chronic, destructive infection to ensure appropriate management and prolonged antibiotic therapy. Good communication with the laboratory is essential, especially in chronic often polymicrobial infections, to ensure that the isolation of one pathogen does not hinder the isolation of others.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
No funding was received for this study.



