-
PDF
- Split View
-
Views
-
Cite
Cite
Mickaela J Nixon, Thomas Grant, Subacute fat embolism syndrome in a young female trauma patient during COVID-19, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab485, https://doi.org/10.1093/jscr/rjab485
Close - Share Icon Share
Abstract
We report the symptom evolution of a young female trauma patient leading to a diagnosis of fat embolism syndrome (FES). Twenty-four hours post-trauma she developed respiratory distress, followed by transient neurological compromise and later petechia. The subtle and fluctuating nature of her presentation made the diagnosis via existing clinical criteria challenging, as did the lack of specificity of thoracic computerized tomography due to the concurrent coronavirus (COVID-19) pandemic. Making the diagnosis was important as it changed the patient’s management, likely preventing a diagnosis in extremis. This case emphasizes the importance of maintaining a high clinical suspicion of FES in any (poly)trauma patient. This is especially true during COVID-19, as correctly identifying non-COVID-19 causes of respiratory failure will prevent additional pandemic victims. In addition, this case supports the need for a diagnostic approach that balances clinical, biochemical and imaging features and takes a cumulative approach in order to identify subacute FES.
INTRODUCTION
Fat embolism syndrome (FES) represents multi-organ compromise due to circulatory fat emboli, classically characterized as a triad of respiratory distress, neurological dysfunction and petechiae [1]. Most commonly seen in trauma patients, long-bone fractures and intramedullary fixations are considered key precipitants of FES [1–3]. FES is commonly conceptualized in its fulminant form; a patient, 12-h post-trauma, presenting with the clinical triad [2, 4]. However, subacute FES is more prevalent but less apparent, leading it to be a commonly overlooked cause of respiratory distress [3, 4]. A lack of definitive diagnostic test, presentation heterogeneity, numerous similarly-presenting differential diagnoses and multiple clinical diagnostic criteria make diagnosing subacute FES difficult.
CASE REPORT
A previously well 15-year-old female arrived to hospital 2 h after being involved in a motorbike collision. She arrived stable with an 8 l.min−1 oxygen requirement but a GCS of 15 and no neurology. Her oxygen requirement settled to 2 l.min−1 shortly thereafter.
Trauma imaging showed fractures of the occipital condyle, pubic rami and midshaft femur. Computerized tomography (CT) images showed no acute intracranial pathology but two foci of ground-glass opacification in the lung, interpreted as mild contusions or early coronavirus disease (COVID-19). Her initial severe acute respiratory syndrome coronavirus 2 (SARS-COV-2) RNA-PCR test was indeterminate.
On Day 1 post-injury, she had an increased oxygen requirement (5 l.min−1), poor air entry bilaterally and poor urine output (UO), but remained alert and oriented; with her chest radiograph (CXR) showing mild perihilar bronchial wall thickening, curtailing concerns of a pneumothorax. A repeat SARS-COV-2 test was negative.
On Day 2, she deteriorated with new fluctuating consciousness in addition to a persistent oxygen requirement and poor UO. A head CT showed no new pathology, but a thoracic CT showed infective features (Fig. 1). A further negative SARS-COV-2 test was returned, so a course of intravenous antibiotics was prescribed. Half a day later, her GCS stabilized.
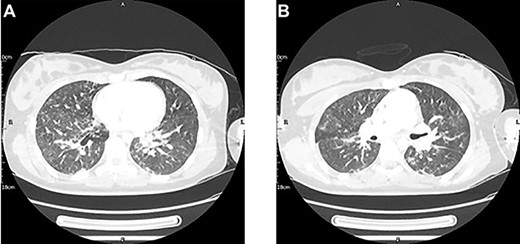
A thoracic CT with contrast conducted 2 days post-injury showed new mild bilateral peri-bronchial wall thickening and bilateral patchy ground-glass changes with early nodular consolidative changes. These features were thought to be in keeping with an infection, either aspirational pneumonia or COVID-19. Pulmonary contusions could have also contributed to the pulmonary changes given the clinical context.
On Day 3, she underwent surgical fixation of her fractured femur. Pre-operative complications included desaturation events en route to, and within, the anaesthetics room. Here, axillary petechiae were noted and fat globules were visible within an arterial blood sample (ABG, Fig. 2). A diagnosis of FES was made, and the anaesthetic and surgical plan subsequently adjusted to preclude clinical deterioration. A femoral but not a sciatic nerve block was sited in order to avoid moving the affected limb. The femur was fixed with a plate, chosen to avoid exacerbating the release of fat emboli, in contrast to an intramedullary nail [5].
Extubation failed one day post-operation, with imaging showing worsening ground-glass and consolidative change. Definitive extubation occurred on Day 6, after which she consistently maintained her oxygen saturations on room air. A Day 7 CXR showed improvement of the bilateral patchy airspace opacification. On Day 9, our patient was stepped down from the paediatric high dependency unit, with a radiograph on Day 10 showing an intact right femur with a mild displacement of comminuted fragments and no peri-prosthetic complications.
On Day 26, she was discharged from hospital, mobilizing with a frame and touch weight bearing on her right leg. Her extended stay was due to complications with the cervical-thoracic orthosis (i.e. Halo) initially applied on Day 3 to stabilise her occipital condyle fracture displacement. The loosening of Halo pins required a return to theatre on Day 14 and again on Day 16 for removal of the Halo in favour of a Miami J collar. On follow-up 5 months post-discharge, the patient had no gait abnormality, no focal neurology and satisfactory alignment on spinal radiograph, the latter to be confirmed by CT.
DISCUSSION
The majority of the clinical criteria used to diagnose FES are rooted in the classic triad but importantly do not require it for a diagnosis (Table 1). In our patient, a diagnosis of FES could have been made based on clinical criteria in advance of her arrival to the anaesthetics room. On Day 1, her hypoxia, tachycardia, oliguria and nearly 20% drop in haemoglobin and 50% drop in platelets since admission meant she had a diagnosis of FES based on the Lindeque Criteria, and a borderline diagnosis based on the Gurd and Modified Gurd Criteria. However, an ABG was required to make a formal diagnosis, with fundoscopy for completeness. Early on Day 2, a different clinical picture with new fluctuating consciousness, diffuse CXR features and tachycardia satisfied the Schonfeld Criteria. Later on Day 2, despite GCS stabilization, our patient’s persistent hypoxia, thrombocytopenia and anaemia, plus new pyrexia and recurring tachypnoea and tachycardia, were suggestive of FES based on the Gurd and Modified Gurd Criteria. By the end of Day 2, and prior to the development of the triad, our patient had demonstrated two of three major Gurd and Modified Gurd criteria, all five of the minor Modified Gurd criteria investigated, and six of the seven Schonfeld criteria.
Numerous diagnostic criteria exist for Fat Embolism Syndrome however, they have faced criticism for lack of sufficient specificity and/or validation [6]
| Gurd and Wilson Criteria [2, 3, 5, 6] . | Modified Gurd Criteria [6] . | Schonfeld Criteria [5, 6, 9] . | Lindeque Criteria [5, 10] . |
|---|---|---|---|
| Major criteria | Petechiae = 5 points | Sustained PaO2 < 60 mmHg kPa | |
| Hypoxaemia (PaO2 < 60 mmHg) | PaO2 < 60 mmHg at FiO2 21% with or without pulmonary infiltrate on chest radiograph | Diffuse alveolar infiltrates on chest radiograph = 4 points | Sustained PCO2 > 55 mmHg or pHa <7.3 |
| Confusion/ altered mentation | Altered mentation with multiple cerebral white matter lesions on MRI | Hypoxaemia (PaO2 < 70 mmHg) = 3 points | Sustained respiratory rate > 35 breaths per minute despite adequate sedation |
| Petechiae | Petechiae on conjunctiva and upper trunk | Fever (>38°C) = 1 point | Increased work of breathing (e.g. dyspnoea, tachycardia, accessory muscle use, etc.) |
| Minor criteria | Tachycardia (>120 beats per minute) = 1 point | ||
| Pyrexia | Pyrexia (>38°C) | Tachypnoea (>30 breaths per minute) = 1 point | |
| Tachycardia | Tachycardia (>100 beats per minute) | Mental confusion = 1 point | |
| Retinal changes (emboli or petechiae on fundoscopy) | Retinal changes (emboli) | ||
| Anuria or oliguria | Auria or oliguria | ||
| Unexplained anaemia (haemoglobin drop of >20%) | Anaemia with coagulopathy or disseminated intravascular coagulopathy without bleeding site | ||
| Thrombocytopenia (drop of >50%) | Thrombocytopaenia, <100 x 109 per litre | ||
| High ESR (>71 mm per hour) | |||
| Fat macroglobulinemia or fat globules in urine or sputum | |||
| Jaundice | |||
| Requirement for diagnosis: | |||
| 2 major (or) 1 major and 4 minor criteria | 2 major and 2 minor (or) 1 major and 3 minor criteria | 5 of more points, over the first 3 days in hospital | 1 or more in a patient with a long bone fracture(s) |
| Gurd and Wilson Criteria [2, 3, 5, 6] . | Modified Gurd Criteria [6] . | Schonfeld Criteria [5, 6, 9] . | Lindeque Criteria [5, 10] . |
|---|---|---|---|
| Major criteria | Petechiae = 5 points | Sustained PaO2 < 60 mmHg kPa | |
| Hypoxaemia (PaO2 < 60 mmHg) | PaO2 < 60 mmHg at FiO2 21% with or without pulmonary infiltrate on chest radiograph | Diffuse alveolar infiltrates on chest radiograph = 4 points | Sustained PCO2 > 55 mmHg or pHa <7.3 |
| Confusion/ altered mentation | Altered mentation with multiple cerebral white matter lesions on MRI | Hypoxaemia (PaO2 < 70 mmHg) = 3 points | Sustained respiratory rate > 35 breaths per minute despite adequate sedation |
| Petechiae | Petechiae on conjunctiva and upper trunk | Fever (>38°C) = 1 point | Increased work of breathing (e.g. dyspnoea, tachycardia, accessory muscle use, etc.) |
| Minor criteria | Tachycardia (>120 beats per minute) = 1 point | ||
| Pyrexia | Pyrexia (>38°C) | Tachypnoea (>30 breaths per minute) = 1 point | |
| Tachycardia | Tachycardia (>100 beats per minute) | Mental confusion = 1 point | |
| Retinal changes (emboli or petechiae on fundoscopy) | Retinal changes (emboli) | ||
| Anuria or oliguria | Auria or oliguria | ||
| Unexplained anaemia (haemoglobin drop of >20%) | Anaemia with coagulopathy or disseminated intravascular coagulopathy without bleeding site | ||
| Thrombocytopenia (drop of >50%) | Thrombocytopaenia, <100 x 109 per litre | ||
| High ESR (>71 mm per hour) | |||
| Fat macroglobulinemia or fat globules in urine or sputum | |||
| Jaundice | |||
| Requirement for diagnosis: | |||
| 2 major (or) 1 major and 4 minor criteria | 2 major and 2 minor (or) 1 major and 3 minor criteria | 5 of more points, over the first 3 days in hospital | 1 or more in a patient with a long bone fracture(s) |
Numerous diagnostic criteria exist for Fat Embolism Syndrome however, they have faced criticism for lack of sufficient specificity and/or validation [6]
| Gurd and Wilson Criteria [2, 3, 5, 6] . | Modified Gurd Criteria [6] . | Schonfeld Criteria [5, 6, 9] . | Lindeque Criteria [5, 10] . |
|---|---|---|---|
| Major criteria | Petechiae = 5 points | Sustained PaO2 < 60 mmHg kPa | |
| Hypoxaemia (PaO2 < 60 mmHg) | PaO2 < 60 mmHg at FiO2 21% with or without pulmonary infiltrate on chest radiograph | Diffuse alveolar infiltrates on chest radiograph = 4 points | Sustained PCO2 > 55 mmHg or pHa <7.3 |
| Confusion/ altered mentation | Altered mentation with multiple cerebral white matter lesions on MRI | Hypoxaemia (PaO2 < 70 mmHg) = 3 points | Sustained respiratory rate > 35 breaths per minute despite adequate sedation |
| Petechiae | Petechiae on conjunctiva and upper trunk | Fever (>38°C) = 1 point | Increased work of breathing (e.g. dyspnoea, tachycardia, accessory muscle use, etc.) |
| Minor criteria | Tachycardia (>120 beats per minute) = 1 point | ||
| Pyrexia | Pyrexia (>38°C) | Tachypnoea (>30 breaths per minute) = 1 point | |
| Tachycardia | Tachycardia (>100 beats per minute) | Mental confusion = 1 point | |
| Retinal changes (emboli or petechiae on fundoscopy) | Retinal changes (emboli) | ||
| Anuria or oliguria | Auria or oliguria | ||
| Unexplained anaemia (haemoglobin drop of >20%) | Anaemia with coagulopathy or disseminated intravascular coagulopathy without bleeding site | ||
| Thrombocytopenia (drop of >50%) | Thrombocytopaenia, <100 x 109 per litre | ||
| High ESR (>71 mm per hour) | |||
| Fat macroglobulinemia or fat globules in urine or sputum | |||
| Jaundice | |||
| Requirement for diagnosis: | |||
| 2 major (or) 1 major and 4 minor criteria | 2 major and 2 minor (or) 1 major and 3 minor criteria | 5 of more points, over the first 3 days in hospital | 1 or more in a patient with a long bone fracture(s) |
| Gurd and Wilson Criteria [2, 3, 5, 6] . | Modified Gurd Criteria [6] . | Schonfeld Criteria [5, 6, 9] . | Lindeque Criteria [5, 10] . |
|---|---|---|---|
| Major criteria | Petechiae = 5 points | Sustained PaO2 < 60 mmHg kPa | |
| Hypoxaemia (PaO2 < 60 mmHg) | PaO2 < 60 mmHg at FiO2 21% with or without pulmonary infiltrate on chest radiograph | Diffuse alveolar infiltrates on chest radiograph = 4 points | Sustained PCO2 > 55 mmHg or pHa <7.3 |
| Confusion/ altered mentation | Altered mentation with multiple cerebral white matter lesions on MRI | Hypoxaemia (PaO2 < 70 mmHg) = 3 points | Sustained respiratory rate > 35 breaths per minute despite adequate sedation |
| Petechiae | Petechiae on conjunctiva and upper trunk | Fever (>38°C) = 1 point | Increased work of breathing (e.g. dyspnoea, tachycardia, accessory muscle use, etc.) |
| Minor criteria | Tachycardia (>120 beats per minute) = 1 point | ||
| Pyrexia | Pyrexia (>38°C) | Tachypnoea (>30 breaths per minute) = 1 point | |
| Tachycardia | Tachycardia (>100 beats per minute) | Mental confusion = 1 point | |
| Retinal changes (emboli or petechiae on fundoscopy) | Retinal changes (emboli) | ||
| Anuria or oliguria | Auria or oliguria | ||
| Unexplained anaemia (haemoglobin drop of >20%) | Anaemia with coagulopathy or disseminated intravascular coagulopathy without bleeding site | ||
| Thrombocytopenia (drop of >50%) | Thrombocytopaenia, <100 x 109 per litre | ||
| High ESR (>71 mm per hour) | |||
| Fat macroglobulinemia or fat globules in urine or sputum | |||
| Jaundice | |||
| Requirement for diagnosis: | |||
| 2 major (or) 1 major and 4 minor criteria | 2 major and 2 minor (or) 1 major and 3 minor criteria | 5 of more points, over the first 3 days in hospital | 1 or more in a patient with a long bone fracture(s) |
A diagnosis of FES cannot be reliant on the triad. First, less than half, and in some studies as low as 3–4%, of FES cases report the simultaneous presence of the triad [1, 6]. In addition, cutaneous manifestations rarely appear until 3–5 days after the onset of respiratory symptoms [2, 4].
Instead, a more telling clinical picture is the combination of respiratory distress and neurological changes, which in isolation is sufficient for a diagnosis based on the Gurd and Modified Gurd Criteria. Respiratory distress, an early and enduring sign in our patient, is the first occurring sign in 95% of FES cases [1] typically developing hours before other signs [4, 5]. Neurological dysfunction, in the form of transient and non-lateralising states ranging from confusion to coma, occurs in 80–85% of FES patients as early as 10-h post-trigger [4, 6]. Furthermore, a cumulative clinical assessment, as suggested by the Schonfeld Criteria, is likely more instructive than a singular clinical snapshot reliant on concurrent symptomatology. Our patient’s varied and asynchronous presentation reinforces this.
Imaging can be a helpful adjunct to clinical assessment of FES. Thoracic CT has shown diagnostic potential, with patchy ground-glass opacities with areas of sparing plus smooth interlobular septal thickening a consistent radiological features in FES [1, 2]. However, an initial negative CT does not rule out FES, and one must consider the consequences of repeated radiological exposure in a young female patient such as ours [2]. In addition, specificity is currently hindered by COVID-19. More promising is cerebral MRI, with the presence of dispersed hyperintense lesions on T2-weighted images found to be sensitive for FES in patients with cerebral involvement, even in those with a normal CT like our patient [2, 5, 6]. Cerebral involvement is not reliant on paradoxical emboli, and radiological signs can appear as early as 4 h post trigger [2, 5–7].
This case captures the difficulties in diagnosing subacute FES and the importance of maintaining a high index of suspicion, especially during COVID-19. Practically, it reminds us of the importance of accounting for the cumulative clinical state, specifically noting a combination of respiratory and neurological features, whilst avoiding reliance on the triad to aid prompt diagnosis. Also, it highlights how the clinical diagnosis of FES can be supported by purposeful use of ABG, fundoscopy, thoracic CT and cerebral MRI.
ACKNOWLEDGMENTS
The authors would like to thank Mr J McMaster for his guidance and for providing the arterial sample photograph, and Dr N Suarez for providing the radiological images.
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest expressed by any of the authors.
FUNDING
No funding was required for this manuscript.
CONSENT
Published with the informed consent of the patient and their parent. Copies of the written consent available on request.
GUARANTOR
TG is nominated as the manuscript’s guarantor.



![Fat macroglobulinemia visualised in an arterial blood gas sample pre-operatively. A common misconception, the presence of fat particles alone is non-specific [2, 6] and considered insufficient to diagnose FES [7, 8].](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2021/10/10.1093_jscr_rjab485/1/m_rjab485f2.jpeg?Expires=1773831108&Signature=PSymuarIZjAJyB2-okKee25gwXIklPYPrLxygrP8DI8dmP~EqYUFgCyg1uWGuoj6V6QdoVqtwoFry8lNzvr4efZhWdEwT11Pyqt0tf-N-ZKl4SNXzQCnhFocIKhCcQrk-Bqy7Rf00Vxa01qn~WJsX6OeL-oFN564Um78fGhPYBwxALea8DezBUV82ecJzeejvTyXHped5gCX7PWw9SjkjGh3Uogx~~wDLInUPAjq2cWG48dXMIBrDIfHEISF9ZTKm4043IUNkuHGnDD~1JiJctniB45OwjqKAyoXkWYae5mbRtENcbsIBG7FCaw2qIkH8Td8RsEfFfqcgO1qZulQKQ__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
