-
PDF
- Split View
-
Views
-
Cite
Cite
Charles C Horn, Liane Wong, Brook S Shepard, William F Gourash, Bryan L McLaughlin, Lee E Fisher, Bestoun H Ahmed, Surgical placement of customized abdominal vagus nerve stimulating and gastrointestinal serosal surface recording electrodes, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab463, https://doi.org/10.1093/jscr/rjab463
Close - Share Icon Share
Abstract
Bioelectronic medical approaches to control vagus nerve-to-organ signaling have the potential to treat cardiac, respiratory, gastrointestinal (GI) and metabolic diseases, such as obesity. Unlike cervical vagus nerve stimulation (VNS), abdominal VNS could provide specific therapeutic control of the GI tract without off-target effects on thoracic organs; however, surgical approaches for abdominal VNS electrode placement are not well established. Moreover, optimal device configurations and additional placement of GI recording electrodes for closed-loop control are largely unknown. We designed VNS cuff and GI planar serosal electrodes and tested placement of these devices in laparoscopic surgery in two cadavers. We determined that electrode positioning on the ventral abdominal vagus nerve and gastric antrum was feasible but other sites, such as the duodenum and proximal stomach, were more difficult. The current investigation can guide potential placement and design of VNS cuff and GI electrodes for development of closed-loop GI therapeutic devices.
INTRODUCTION
Cervical vagus nerve stimulation (VNS) was approved by the Food and Drug Administration (FDA) for the treatment of drug-resistant epilepsy in 1997 and depression in 2005; >100 000 patients have been implanted [1]. Emerging literature indicates the potential of cervical VNS for treatment of additional diseases, such as rheumatoid arthritis and inflammatory bowel disease [2–4]; however, this approach is largely non-specific because the cervical vagus nerve contains afferent and efferent fibers from nearly all thoracic and abdominal organs.
More specificity can be achieved by applying VNS close to the target organ; for example, vBloc therapy was FDA approved in 2015 for treatment of obesity and places electrodes on the gastric branches of the vagus nerve. There are, however, several limitations to the vBloc approach including: (i) distal gastric nerve branches can have significant anatomical variability and (ii) vBloc is open-loop stimulation using stimulation parameters to produce unknown biological outcomes [5]. A more effective approach could be placement of a nerve cuff on the abdominal vagus nerve trunk and, also, monitoring gastrointestinal (GI) responses with additional recording electrodes to provide closed-loop control [6].
In the current study we designed vagus nerve cuff and GI serosal surface planar electrodes for implantation into humans based on designs tested in our preclinical model [6, 7]. We then tested the surgical access and placement of these devices in two cadavers.
CASE REPORT
The current study was approved by the Committee for Oversight of Research and Clinical Training Involving Decedents (CORID) at the University of Pittsburgh (Application #1017). For laparoscopic placement, precision nerve cuffs were designed and molded with a unique self-closing mechanism and manufactured using medical-grade silicone and platinum–iridium. Surface electrodes were produced using a seamless, fusion-bonded silicone substrate containing reinforcement fibers for suturing and platinum–iridium (90/10) electrodes (Fig. 1). Human cadaver specimens included: (i) female, age = 90 years, body weight = 72.6 kg, height = 155 cm, body mass index (BMI) = 30 and (ii) male, age = 75 years, body weight = 115.7 kg, height = 183 cm, BMI = 35. BMIs of at least 30 were selected because they fall into the obese range of the Centers for Disease Control (USA) and we are developing therapies for the control of obesity. Cadavers were laid on a surgical table at an incline (thorax higher than abdominal cavity) to stretch the stomach away from the diaphragm to facilitate locating the esophagus for VNS cuff placement. Three laparoscopic ports were placed in the abdominal wall to insert instruments and electrodes. The abdominal cavity was filled with CO2 gas to expand the view of the organs. First, the ventral esophagus was located, and a small amount of connective tissue was trimmed from the left vagus nerve trunk before placement of the cuff electrode (Fig. 2). Second, the duodenum was located and two electrodes were placed proximal and distal (Fig. 3). Finally, up to four electrodes were placed on the ventral surface of the stomach (Fig. 3). Surgery was guided by using the video feed from a laparoscopic tower (Stryker Endoscopy; San Jose, CA; Stryker 1588 AIM camera and console L10 LED light source, PneumoSure 45L insufflator, SDC3 Digital Capture, Vision Pro LED monitor), which was also used to collect still images. At the end of each procedure, the abdominal wall was surgically opened, and the GI tract and esophagus were extracted and placed on a tray to image the location of the electrodes (diagrams of these locations are shown in Fig. 3). Timing for each segment of the procedure is indicated in Table 1.
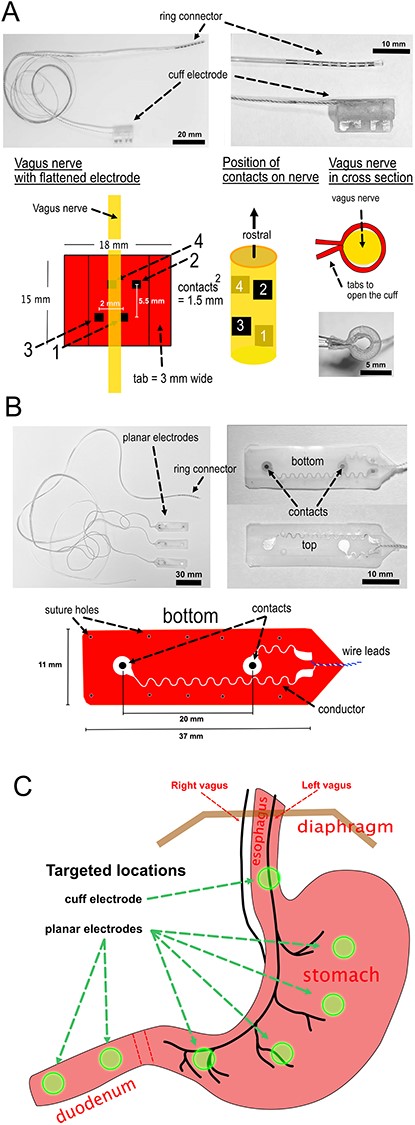
Design of electrodes and planned placement. (A) Vagus nerve cuff electrodes. (B) GI planar electrodes. (C) Planned placement of the cuff and planar electrodes.
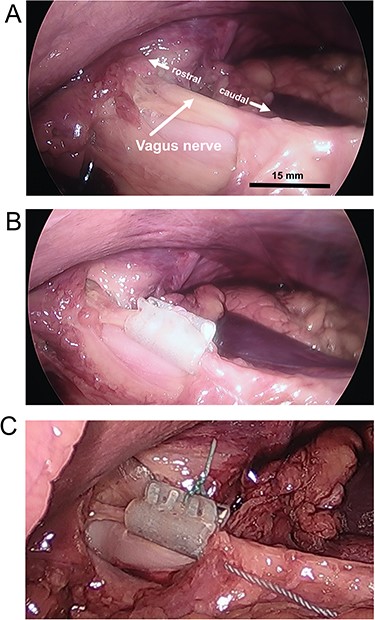
Vagus nerve cuff electrode placements. (A) Ventral vagus nerve trunk. (B) Placement of the cuff electrode. (C) Cuff electrodes with a suture to keep tabs closed. Bar = approximate scale with greater accuracy in the foreground.
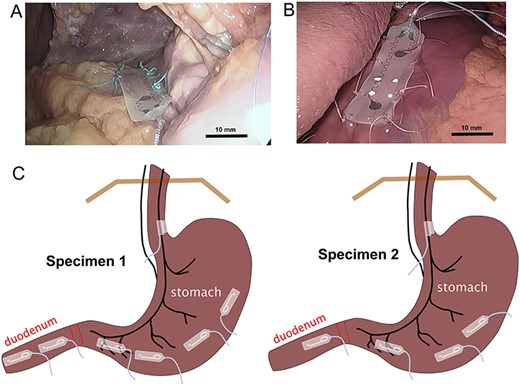
GI planar electrode placements. (A) electrode positioning on duodenum, with initial sutures. (B) electrode on stomach. (C) Diagram of final electrode positions on the stomach (and vagus nerve). Bar = approximate scale with greater accuracy in the foreground.
| . | Specimen 1, female . | Specimen 2, male . |
|---|---|---|
| From first incision to placement of electrodes in abdominal cavity | 10 min | 4 min |
| Locating the left abdominal vagus | 6 min | 6 min |
| Tissue trimming, placement, and suturing of VNS cuff electrode | 32 min | 18 min |
| Suturing of each GI planar electrode, mean (±standard deviation) | 20.5 (±4.2) min | 17.3 (±3.3) mina |
| Total time | 3 h, 30 min | 2 h, 24 min |
| . | Specimen 1, female . | Specimen 2, male . |
|---|---|---|
| From first incision to placement of electrodes in abdominal cavity | 10 min | 4 min |
| Locating the left abdominal vagus | 6 min | 6 min |
| Tissue trimming, placement, and suturing of VNS cuff electrode | 32 min | 18 min |
| Suturing of each GI planar electrode, mean (±standard deviation) | 20.5 (±4.2) min | 17.3 (±3.3) mina |
| Total time | 3 h, 30 min | 2 h, 24 min |
aCalculation is based on only four planar electrodes.
| . | Specimen 1, female . | Specimen 2, male . |
|---|---|---|
| From first incision to placement of electrodes in abdominal cavity | 10 min | 4 min |
| Locating the left abdominal vagus | 6 min | 6 min |
| Tissue trimming, placement, and suturing of VNS cuff electrode | 32 min | 18 min |
| Suturing of each GI planar electrode, mean (±standard deviation) | 20.5 (±4.2) min | 17.3 (±3.3) mina |
| Total time | 3 h, 30 min | 2 h, 24 min |
| . | Specimen 1, female . | Specimen 2, male . |
|---|---|---|
| From first incision to placement of electrodes in abdominal cavity | 10 min | 4 min |
| Locating the left abdominal vagus | 6 min | 6 min |
| Tissue trimming, placement, and suturing of VNS cuff electrode | 32 min | 18 min |
| Suturing of each GI planar electrode, mean (±standard deviation) | 20.5 (±4.2) min | 17.3 (±3.3) mina |
| Total time | 3 h, 30 min | 2 h, 24 min |
aCalculation is based on only four planar electrodes.
DISCUSSION
The current study demonstrated the feasibility of placing VNS and GI planar electrodes using a laparoscopic approach. It was more difficult to accurately place planar electrodes because of the few anatomical landmarks and the curvature of the proximal stomach and duodenum; however, it is possible that this would be easier in a living stomach with blood flow and coloration and with a fasted condition prior to surgery.
There are several limitations of the current study. First, although it was easy to locate the ventral (left) vagus nerve, location of the dorsal trunk was not attempted, and the dorsal vagus could provide better VNS control of GI function based on effects of VNS on GI motility in animal testing [8]. Second, one of the subjects (specimen #1, female; Table 1) had a cholecystectomy and adhesion of tissue obscured the surgical approach on the left side of the body in the region of the duodenum; however, this did not substantially affect the timing for placement of GI electrodes. Third, it is also unclear whether the electrode contact spacing is optimal for recording and stimulation.
Future directions should include: (i) determining the location of the wireless transmitter that would connect to the electrodes; (ii) reducing the trauma of suturing the planar electrodes by using fewer electrodes, e.g. one on the stomach antrum and one on the duodenum, and the potential use of a different attachment method, such as hydrogel [9].
ACKNOWLEDGEMENTS
The authors acknowledge the assistance of Jessica Brooks and Jonathan Harms. We also appreciate the use of the Pittsburgh CREATES facility for performing these procedures.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
This work was supported by National Institutes of Health funding (Common fund SPARC Program award U18TR002205).
References
Nanivadekar AC, Miller DM, Fulton S, Wong L, Ogren J, Chitnis G, et al.
Shulgach JA, Beam DW, Nanivadekar AC, Miller DM, Fulton S, Sciullo M, et al.
Horn CC, Forssell M, Sciullo M, Harms JE, Fulton S, Mou C, et al.



