-
PDF
- Split View
-
Views
-
Cite
Cite
Rahoui Moez, Boulma Rami, Khouni Hassen, Metachronous splenic metastasis from renal cell carcinoma: a case report, Journal of Surgical Case Reports, Volume 2021, Issue 10, October 2021, rjab449, https://doi.org/10.1093/jscr/rjab449
Close - Share Icon Share
Abstract
The causes of isolated solid splenic lesions are wide and varied, and as such can present a diagnostic challenge. Splenic metastases were previously considered exceptionally rare. We report a case of a patient who had isolated splenic metastases with a previous history of left nephrectomy due to a renal cell carcinoma 3 years before. This report represents the first case reported in our country and wants to add to literature one more case of splenic metastasis from renal cell carcinoma.
INTRODUCTION
Renal cell carcinoma (RCC) accounts for 3% of all cancers [1]. It is the third urologic cancer after prostate and bladder cancers. The preferred sites of metastasis are the lungs, bones, liver and brain [2]; however, splenic metastasis from RCC in extremely rare and up to now only few cases has been described in the available literature. We report the case of a men who presented with a splenic lesion 3 years after radical nephrectomy.
CASE REPORT
We report the case of 47-year-old men, with a chronic renal failure, who had been operated on 3 years previously for a total left radical nephrectomy for clear cell renal cancer of stage pT3G1N0M0. During the follow-up of the patient, a routine cancer screening with ultrasound revealed a mass in the spleen of about 4 cm of diameter. Laboratory investigations were normal apart from known renal failure (Table 1). Due to known chronic kidney disease and allergy to Iodinated contrast media, an abdominal magnetic resonance imaging (MRI) confirmed the presence of a splenic lesion (Fig. 1). Based on these radiological findings, the splenic mass was diagnosed as a suspicious metastatic lesion. An open splenectomy was performed. The patient recovered uneventfully and was discharged 5 days after surgery. He received pneumococcal, meningococcal and Haemophilus influenzae vaccine. Histologic analysis of the lesion confirmed the presence of clear cell renal cancer metastasis (Fig. 2). The patient was referred to the oncology department for adjuvant treatment with sunitinib. His follow up consisted on abdominal ultrascan (US) every 3 months and MRI at 6 and 12 months from surgery. After 36 months, the patient is doing well with no signs of tumor recurrence.
| Biochemical and hematological parameters . | Value . |
|---|---|
| Hemoglobin (g/dl) | 12.4 |
| White blood cells | 8700 |
| Platelets | 278 000 |
| Serum creatinine (umol/L) | 175 |
| Calcium (mmol/L) | 2.3 |
| Albumin (g/L) | 37 |
| Biochemical and hematological parameters . | Value . |
|---|---|
| Hemoglobin (g/dl) | 12.4 |
| White blood cells | 8700 |
| Platelets | 278 000 |
| Serum creatinine (umol/L) | 175 |
| Calcium (mmol/L) | 2.3 |
| Albumin (g/L) | 37 |
| Biochemical and hematological parameters . | Value . |
|---|---|
| Hemoglobin (g/dl) | 12.4 |
| White blood cells | 8700 |
| Platelets | 278 000 |
| Serum creatinine (umol/L) | 175 |
| Calcium (mmol/L) | 2.3 |
| Albumin (g/L) | 37 |
| Biochemical and hematological parameters . | Value . |
|---|---|
| Hemoglobin (g/dl) | 12.4 |
| White blood cells | 8700 |
| Platelets | 278 000 |
| Serum creatinine (umol/L) | 175 |
| Calcium (mmol/L) | 2.3 |
| Albumin (g/L) | 37 |
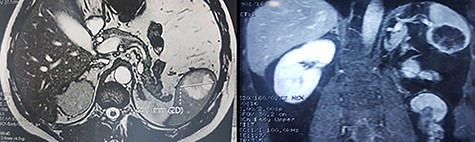
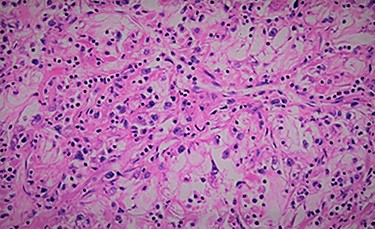
DISCUSSION
Excluding hematological diseases, primary and metastatic tumors to the spleen are uncommon. The reported incidence of metastatic tumors in spleen varies from 0.3% to 7.3%, but it is generally linked to hematological malignancies [2]. Metastasis to the spleen is infrequent. The most common primary cancers are lung cancer, skin malignant melanoma and breast cancer [3]. About 30% of the patients with renal cell carcinoma already have distant metastasis at the time of diagnosis. Splenic metastasis from RCC in extremely rare and up to now only few cases has been described in the available literature [4] (Table 2). The incidence of isolated splenic metastasis from any given primary is also particularly rare. Generally, metastases are asymptomatic and discovered during the follow up with US and computed tomography (CT).
| Authors . | Age/sex . | Metastasing time . | Primary . | Outcome . |
|---|---|---|---|---|
| Strum | 59 y/M | 22 y | Right | Dead (5 mo) |
| Ishida et al. | 50 y/M | 7 y | Left | Alive (6 y) |
| Nabi et al. | 50 y/f | (Synchronus) | Left | Alive (6 mo) |
| Kugel et al. | 72 y/M | 2 y | Left | Dead (1 y) |
| McGregor et al. | 65 y/M | (Synchronus) | Left | ND |
| Shuck-Bello et al. | 74 y/M | 15 y | Right | ND |
| Ielpo et al. | 82 y/M | 14 y | Left | Alive (1 y 3 mo) |
| Moir et al. | 70 y/F | 11 mo | Left | Alive (2y) |
| Nunes et al. | 60 y/f | 5 y | Left | Alive (6 mo) |
| Hardikar | 29 y/M | (Synchronus) | Left | Alive (2 y) |
| Zhang et al. | 67 y/M | 2 y | Left | Alive (5 mo) |
| Grewal et al. | 53 y/m | 2 mo | Left | ND |
| Liu et al. | 75 y/M | (Synchronus) | Right | Alive (1 y 4 mo) |
| Ramao et al. | 48 y/M | 11 y | Left | Alive (2 mo) |
| Rahoui, 2021 | 47 y/M | 3 y | Left | Alive (3 y) |
| Authors . | Age/sex . | Metastasing time . | Primary . | Outcome . |
|---|---|---|---|---|
| Strum | 59 y/M | 22 y | Right | Dead (5 mo) |
| Ishida et al. | 50 y/M | 7 y | Left | Alive (6 y) |
| Nabi et al. | 50 y/f | (Synchronus) | Left | Alive (6 mo) |
| Kugel et al. | 72 y/M | 2 y | Left | Dead (1 y) |
| McGregor et al. | 65 y/M | (Synchronus) | Left | ND |
| Shuck-Bello et al. | 74 y/M | 15 y | Right | ND |
| Ielpo et al. | 82 y/M | 14 y | Left | Alive (1 y 3 mo) |
| Moir et al. | 70 y/F | 11 mo | Left | Alive (2y) |
| Nunes et al. | 60 y/f | 5 y | Left | Alive (6 mo) |
| Hardikar | 29 y/M | (Synchronus) | Left | Alive (2 y) |
| Zhang et al. | 67 y/M | 2 y | Left | Alive (5 mo) |
| Grewal et al. | 53 y/m | 2 mo | Left | ND |
| Liu et al. | 75 y/M | (Synchronus) | Right | Alive (1 y 4 mo) |
| Ramao et al. | 48 y/M | 11 y | Left | Alive (2 mo) |
| Rahoui, 2021 | 47 y/M | 3 y | Left | Alive (3 y) |
ND: not described.
| Authors . | Age/sex . | Metastasing time . | Primary . | Outcome . |
|---|---|---|---|---|
| Strum | 59 y/M | 22 y | Right | Dead (5 mo) |
| Ishida et al. | 50 y/M | 7 y | Left | Alive (6 y) |
| Nabi et al. | 50 y/f | (Synchronus) | Left | Alive (6 mo) |
| Kugel et al. | 72 y/M | 2 y | Left | Dead (1 y) |
| McGregor et al. | 65 y/M | (Synchronus) | Left | ND |
| Shuck-Bello et al. | 74 y/M | 15 y | Right | ND |
| Ielpo et al. | 82 y/M | 14 y | Left | Alive (1 y 3 mo) |
| Moir et al. | 70 y/F | 11 mo | Left | Alive (2y) |
| Nunes et al. | 60 y/f | 5 y | Left | Alive (6 mo) |
| Hardikar | 29 y/M | (Synchronus) | Left | Alive (2 y) |
| Zhang et al. | 67 y/M | 2 y | Left | Alive (5 mo) |
| Grewal et al. | 53 y/m | 2 mo | Left | ND |
| Liu et al. | 75 y/M | (Synchronus) | Right | Alive (1 y 4 mo) |
| Ramao et al. | 48 y/M | 11 y | Left | Alive (2 mo) |
| Rahoui, 2021 | 47 y/M | 3 y | Left | Alive (3 y) |
| Authors . | Age/sex . | Metastasing time . | Primary . | Outcome . |
|---|---|---|---|---|
| Strum | 59 y/M | 22 y | Right | Dead (5 mo) |
| Ishida et al. | 50 y/M | 7 y | Left | Alive (6 y) |
| Nabi et al. | 50 y/f | (Synchronus) | Left | Alive (6 mo) |
| Kugel et al. | 72 y/M | 2 y | Left | Dead (1 y) |
| McGregor et al. | 65 y/M | (Synchronus) | Left | ND |
| Shuck-Bello et al. | 74 y/M | 15 y | Right | ND |
| Ielpo et al. | 82 y/M | 14 y | Left | Alive (1 y 3 mo) |
| Moir et al. | 70 y/F | 11 mo | Left | Alive (2y) |
| Nunes et al. | 60 y/f | 5 y | Left | Alive (6 mo) |
| Hardikar | 29 y/M | (Synchronus) | Left | Alive (2 y) |
| Zhang et al. | 67 y/M | 2 y | Left | Alive (5 mo) |
| Grewal et al. | 53 y/m | 2 mo | Left | ND |
| Liu et al. | 75 y/M | (Synchronus) | Right | Alive (1 y 4 mo) |
| Ramao et al. | 48 y/M | 11 y | Left | Alive (2 mo) |
| Rahoui, 2021 | 47 y/M | 3 y | Left | Alive (3 y) |
ND: not described.
The definitive diagnosis is based on a histological examination. The cellular diagnosis can be made by using percutaneous biopsy (using US or CT guidance) [5, 6] or endoscopic fine needle aspiration [7]. Surgery for splenic metastasis of kidney cancer is recommended for a palliative purpose and prevention of future complications. In these cases, an adjuvant therapy is recommended but it is effective in only about 10% of patients [8]. The prognosis, if the metastases are multiple is unfavorable, while if it is isolated the surgery, is the best treatment. If a complete resection of the metastasis is achieved the prognosis is favorable [9]. Our patient underwent an open splenectomy without morbidity and was referred to the oncology department for adjuvant treatment with sunitinib. Three years the patient is doing well with no signs of tumor recurrence.
CONCLUSION
Splenic metastasis from RCC in extremely rare. They are often asymptomatic and generally discovered on surveillance imaging. The optimal treatment is based on splenectomy to avoid any complications. The adjuvant treatment should not be considered a standard of care outside from clinical trials.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The approval of the current study has been granted by the medical committee of research ethics of FSI hospital . Written informed consent was obtained from the patient for publication of this study. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
CONSENT FOR PUBLICATION
Written informed consent was obtained from the patient for publication of this study. A copy of the written consent is available for review by the Editor on request.
FUNDING
No funding was received.
COMPETING INTERESTS
The authors declare that they have no competing interests.



