-
PDF
- Split View
-
Views
-
Cite
Cite
Alex Mremi, Doris Rwenyagila, Kondo Chilonga, Adnan Sadiq, David Msuya, Jay Lodhia, Immature teratoma of the ovary in a 1 year and 9-month-old child: a case report and review of the literature, Journal of Surgical Case Reports, Volume 2021, Issue 1, January 2021, rjaa609, https://doi.org/10.1093/jscr/rjaa609
Close - Share Icon Share
Abstract
Immature teratoma of the ovary is a rare malignant germ cell tumor whose etiology is unknown. Preoperative diagnosis and treatment of this tumor can be challenging for clinicians. We present a 1-year and 9-month female child who presented with a 1- month history of progressive nontender abdominal distension. Computed tomography scan of the abdomen revealed a huge well-defined heterogenous mass arising from the peritoneal cavity. Surgical resection was performed. Histopathology coupled with immunohistochemical analysis of the specimen confirmed it to be an ovarian immature teratoma, grade one. The child recovered well postoperatively. Surgery alone is curative for most children and adolescents with resectable ovarian immature teratoma thus avoiding the long-term effects of chemotherapy in most children with this disease.
INTRODUCTION
The immature teratoma of the ovary is a rare malignant germ cell tumor whose etiology is unknown. It comprises <1% of teratomas of the ovary [3]. The tumor is composed of tissue derived from the three germ layers-ectoderm, mesoderm and endoderm; contrary to the much more frequent mature teratoma, it contains immature or embryonal structures [10]. This case report presents a Tanzanian child with abdominal mass that was confirmed to be immature teratoma. The overview of the literature is also provided.
CASE PRESENTATION
One year and 9-month old female baby was referred to us due to a sudden onset of abdominal distension which was progressive in nature. Her mother reported that this presentation was of 1-month duration and was associated with intermitted low-grade fevers. The mother reported that the child was not irritable, not in pain and denied history of early satiety or change in bowel habits. Urine output was reported to be normal with normal color and no loss of weight. Past medical history was not eventful and the mother denied any familial history of malignancies. The child’s immunization and developmental milestones were reported to be normal.
Upon examination, the child was ill looking, had mild conjuctival palor, not jaundiced and had no regional lymphadenopathy. Axillary temperature was 37°C, pulse rate of 149 bpm, respiratory rate of 27 breaths per minute and was saturating at 97% on room air. The abdomen was grossly distended with visible superficial veins, flat umbilicus, tense but not tender and bowel sounds were heard. Other systems were unremarkable. The complete blood count showed leukocytosis of 25.2 × 109/L, microcytic hypochromic anemia of 9.0 g/dl and thrombocytosis of 872 × 109/L. Liver enzymes, electrolytes and serum calcium were in normal range, serum creatinine was 20 μmol/L and lactate dehydrogenase of 673.47 U/L. the child was transfused with 200 ml of whole blood.
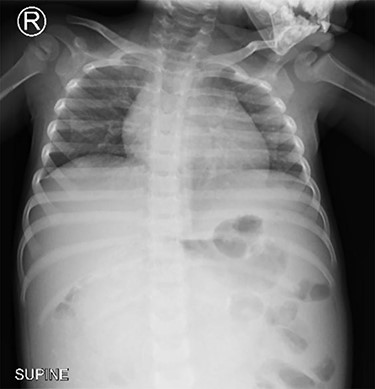
Chest x-ray (Fig. 1) was normal, while abdominal computed tomography (CT) scan showed a huge well-defined heterogenous mass arising from the peritoneal cavity measuring 10.3 cm (AP) × 16.2 cm (T) × 16.7 cm (CC) with central and peripheral calcifications, (Fig. 2). The tumor had pressure effect on the bowels, pancreas and right kidney. The child underwent an elective laparotomy where intraoperatively amber colored ascites was found and a huge left ovarian mass that was free and not adherent to adjacent structures. Left sided salpingo-ophorectomy was done removing the whole mass (Fig. 3) and was sent for histopathology analysis. The uterus, right ovary and fallopian tube were essentially unremarkable. Histopathology of the specimen highlighted a variety of immature and mature tissues derived from the three germ layers (Fig. 4 A–C); whereas a minimal panel of immunohistochemical staining demonstrated strong positivity of the tumor cells for S100 and Neuron specific enolase antibodies (Fig. 4D); thus confirming the diagnosis of immature teratoma, low grade (Grade I), pT2cN0M0. After establishing the diagnosis, the child was reviewed in tumor board by a multidisciplinary team of experts. She recovered well and was discharged on the fourth day postoperatively post discharge; the child attended our pediatric oncology clinic with no any new complaints. She was followed-up with serum chorionic gonadotropin (HCG) and alpha-feto protein (AFP) tests which were within normal during the two monthly visits.
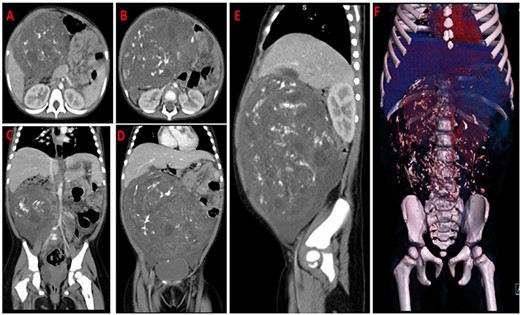
CT-scan images of axial (A,B), coronal (C,D), sagittal contrasted abdomen (E) and 3D virtual reconstruction (F); showing a large heterogenous mass occupying the abdomen and pelvis with mixed fat and soft tissue density and intratumoral calcifications displacing the air filled bowels to the left side.

Gross appearance of the ovarian mass demonstrating an oval shape and variegated cut surface with hemorrhage and necrosis.
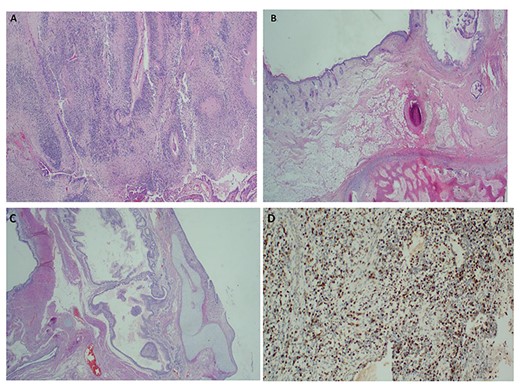
Histopathology of the immature ovarian teratoma demonstrating both immature neuroepithelial and mesenchymal elements ×40 H&E (A–C); and positive immunostaining of the immature neuroepithelium with S100 × 100 IHC, (D).
DISCUSSION
The word ‘teratoma’ is derived from Greek work ‘teraton’ meaning monster [9]. Immature teratoma of the ovary is a malignant germ cell tumor containing variable amounts of immature tissue, typically primitive neuroectodermal [9]. The etiology for this disease entity is usually unknown [9]. Although benign cystic teratomas of the ovary are relatively common tumor that can occur at any age, the incidence of malignant elements in a teratoma is low (~1–2%) [3, 9, 10]. The tumor occurs in all ages but usually affects mostly young females <20 years and almost unknown after the menopause [5]. In view of this, teratomas occurring in childhood, adolescence and early adult life should always be examined carefully and thoroughly sampled.
Clinically, patients with this tumor usually are asymptomatic until it reaches a considerable size [10]. It tends to grow rapidly and may manifest itself as a pelvic or lower abdominal mass. It may cause pressure symptoms, abdominal heaviness, dull pain, or it may undergo torsion, causing acute abdominal pain [9]. Peritoneal implants and metastases are not infrequently present at operation for the removal of the primary tumor [9, 10]. Immature teratoma usually penetrates its capsule, and forms adhesions to the surrounding structures. It spreads throughout the peritoneal cavity by implantation and metastasizes first to the retroperitoneal, para-aortic, and more distant lymph nodes and later to the lungs, liver and other organs [10]. Our patient however, had neither adhesions nor distant metastasis and the tumor capsule was intact.
Diagnosis of immature teratoma of the ovary can be challenging especially in small biopsies and immunohistochemistry has been reported to have limited role in diagnosis of immature teratomas [9]. With resection specimens, extensive sampling may be needed to include areas of immature teratoma at the time during grossing or with frozen section in cases where a preoperative diagnosis is suspected [7]. Gross examination of immature teratoma specimens usually are large solid mass with necrosis and hemorrhage on cut sections, as it was the case for our patient.
Microscopically, the tumors demonstrate variable amounts of mature elements from all three germ layers, admixed with immature elements, mostly neuroectodermal [7, 8]. Immature neuroepithelium can be spindled (sarcomatoid) or with rosette, pseudorosette and primitive tubule formation, the latter is easier to recognize [7, 9].
Similarly, the tumor cells appear primitive, with scant cytoplasm, hyperchromatic nuclei and frequent mitoses, which help differentiate the immature elements from mature brain tissue (Fig. 4). Immature teratoma must be differentiated from malignant mesodermal mixed tumor, which although occurring most frequently in the uterus, also occurs in the ovary [7, 8].
For the optimal oncological treatment outcome, the management of ovarian immature teratoma in pediatric patients should consist of a multidisciplinary team of neonatologists, neonatal anesthetists, gynecologists with special interest in pediatric gynecology and gynecologic oncologist experts. Current treatment recommendations for immature teratoma include an initial staging procedure for all patients; however, since in most cases the diagnosis of a germ cell malignancy is made post operatively, i.e. on final pathology, reports of more limited surgery are common [4]. Grade is the most important risk factor for relapse across all age groups [2, 10] whereas stage is the most important factor for prognosis, which is greatly improved after chemotherapy [6]. Patients with stage IA, grade 1 tumors have an excellent prognosis, do not require adjuvant treatment and postoperative observation is recommended. Extraovarian disease (tumors that are grade 2 or 3) is an accepted indication for postoperative chemotherapy [6]. Moreover, very immature and poorly differentiated tumors have been found to be associated with worse prognosis, whereas a more favorable outcome has been observed in patients with more mature and better differentiated tumors [1]. Similarly, older age at diagnosis, higher stage and grade confer worse prognosis but overall improved after chemotherapy for stage IV disease [1, 2, 6].
In conclusion, our report highlighted that surgical treatment is likely to be associated with better outcome for pediatric population with immature ovarian teratomas particularly when they present with the disease at early grade and stage.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Written informed consent to participate in this study and subsequent publication for this case report was obtained from the patient’s mother. A copy of the consent is available on record.
AVAILABILITY OF DATA AND MATERIALS
All the information included in the current study is available from the corresponding author upon editorial office request.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
ACKNOWLEDGMENTS
The authors would like to thank the child’s mother for the permission to share her child’s medical information for educational purposes and publication. No funding was received for this report.



