-
PDF
- Split View
-
Views
-
Cite
Cite
Ruben D Salas-Parra, Larissa L Tavares, Ranjan Gupta, Joseph Silletti, Ajay Shah, Dedifferentiated liposarcoma of the scrotum. Case report and literature review, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa342, https://doi.org/10.1093/jscr/rjaa342
Close - Share Icon Share
Abstract
Malignant paratesticular and spermatic cord tumors are rare and often misdiagnosed preoperatively due to clinical presentations similar to other benign scrotal mass etiologies. Only a few cases regarding giant, paratesticular liposarcomas (>10 cm) have been reported. We report a unique case of an aggressive giant dedifferentiated liposarcoma of the scrotum with osteosarcoma features in a 70-year-old patient who initially presented with indolent scrotal swelling. A CT scan showed a large, complex, solid and cystic mass (12.0 x 15.5 x 19.0 cm) in the right scrotum. With a concern of a complex hydrocele, the patient was taken to the operating room for a scrotal approach to excise the hydrocele, but the spermatic cord was not traced to any discernible testicle as the entire mass was indurated and multilocular, and was excised. Pathology revealed a dedifferentiated liposarcoma, with MDM2 amplification. The patient’s course was complicated due to metastatic disease.
INTRODUCTION
Scrotal masses are divided into testicular or paratesticular masses. The majority of paratesticular masses are benign, but malignancies do occur. Liposarcomas are the most common type of paratesticular tumors [1–4]. Their treatment is usually complete surgical resection, but there is no consensus in regards to adjuvant therapies for these aggressive malignancies.
We report on an aggressive case of a giant, dedifferentiated liposarcoma of the scrotum with osteosarcoma features with a complicated respiratory course due to metastatic disease.
CASE REPORT
A 70-year-old African American male presented to the clinic with indolent scrotal swelling for years with an increase in size during the past 3 months. He denied urinary tract symptoms. Examination showed a large right scrotal mass of approximately 25 x 10 cm, with areas consistent with fluid by transillumination, but the right testicle itself was not palpable.
Scrotal ultrasonography revealed a complex septated cystic lesion on the right side of the scrotum (11.2 x 8.9 x 11.6 cm) (Fig. 1). The CT scan showed a large, complex solid and cystic mass (12.0 x 15.55 x 19.0 cm) in the right scrotum; and external to the testicle, a large amount of fat, with no abdominal lymphadenopathies (Fig. 2).
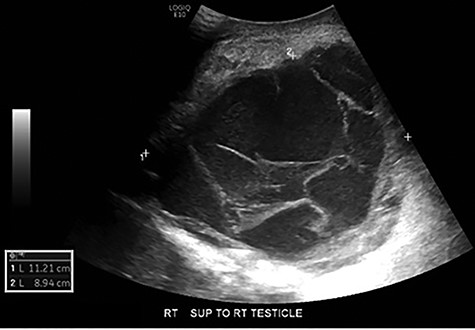
Scrotal ultrasound: complex septated cystic lesion on the right side of the scrotum measuring 11.2 x 8.9 x 11.6 cm. Nonspecific scrotal wall thickening was noted.
Out of a concern for an encrusted hematocele, the patient was taken for scrotal exploration. The spermatic cord, however, could not be traced to any discernible testicle as an indurated and multilocular mass was found. Once the mass was completely freed and excised, the specimen was sent to pathology. The patient did well postoperatively.
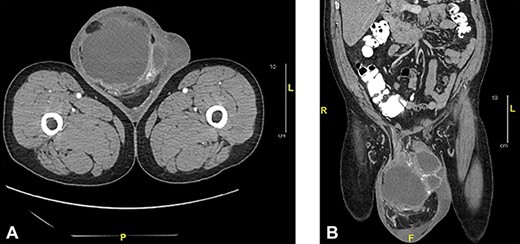
Axial (a) and sagittal (b) CT of abdomen and pelvis with oral and intravenous contrast: showed a large complex cystic and solid mass measuring 12.0 x 15.5 x 19.0 cm in the right scrotum.
Histopathology showed the tumor of 1240 grams and measuring 20 x 16 x 12 cm as a well-differentiated liposarcoma that transitioned to a high-grade non-lipogenic spindle cell sarcoma. The tumor had features of high-grade osteosarcoma (Fig. 3), and was positive for MDM2 amplification consistent with dedifferentiated liposarcoma. Surgical margins were involved.
![Histopathology dedifferentiated liposarcoma (High-grade): areas of well-differentiated liposarcoma comprised of atypical stromal cells and a few lipoblast [x400] (a). Dedifferentiated zones resembling undiferenciated pleomorphic sarcoma. The tumor is comprised of spindle cells with numerous mitotic figures; the cells are arranged in interlacing or storiform patter [x200] (b). Areas of osteosarcoma comprised of bands of stoeid tissue surrounded by pleomorphic spindles-shaped cells [x200] (c).](https://oupdevcdn.silverchair-staging.com/oup/backfile/Content_public/Journal/jscr/2020/9/10.1093_jscr_rjaa342/2/m_rjaa342f3.jpeg?Expires=1770628461&Signature=Ab~UlRU-yePBt0lUEO~Z7oloLs-hkTqHUUpKab~4Sw3qVymUI5uU7efakl1J9sB879qAzH~Hh76T9Fyx~6ME7XYMj6UIKUoilUAMIleNjQd5j6sZa7WZACoNd2yHTapKRb-h4O87iLoIEyI7zUnH5Wf1nPqIsG3a0gpqf8GVeizwIrpbGlgpvrwvljtsmr1znsc3ITJo2UGS3OWBPUhqyu8q2d3coxNWBP2qpQyImgayq6c6V0NorZcSHfHvnruDEwkjGu0oLQwduQpJCewt3lLgE0-GIRA~NIO6Vf-vSI8mKJSbLEDBYDQUtHdkW0tQwt6hLRFc~9qPxkp4leJrsQ__&Key-Pair-Id=APKAIYYTVHKX7JZB5EAA)
Histopathology dedifferentiated liposarcoma (High-grade): areas of well-differentiated liposarcoma comprised of atypical stromal cells and a few lipoblast [x400] (a). Dedifferentiated zones resembling undiferenciated pleomorphic sarcoma. The tumor is comprised of spindle cells with numerous mitotic figures; the cells are arranged in interlacing or storiform patter [x200] (b). Areas of osteosarcoma comprised of bands of stoeid tissue surrounded by pleomorphic spindles-shaped cells [x200] (c).
Chemotherapy was refused by the patient postoperatively. After 6 weeks, the patient returned to the emergency room presenting with shortness of breath, due to left-sided pleural effusion requiring thoracentesis. Cytology was negative for malignancy. He returned 3 weeks later with similar respiratory symptoms. A CT chest showed recurrent left pleural effusion (Fig. 4). Thoracic surgery was consulted and a pleural biopsy with PleurX catheter insertion was performed. Metastatic, dedifferentiated liposarcoma was reported. During his hospital course, the patient developed worsening respiratory failure and died after he was treated with broad-spectrum antibiotics for pneumonia and suspicious COVID-19, which was later reported positive.
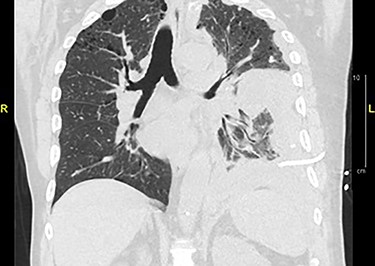
CT chest without contrast: extensive lobulated left-sided pleural thickening with effusion, consistent with metastatic disease.
DISCUSSION
Scrotal masses are a common patient complaint but can prove to be diagnostically challenging. They have a wide variety of benign etiologies, but in rare instances can present as malignant. Most paratesticular tumors arise from the spermatic cord (75%). Liposarcomas (40%) are the most common histologic type, followed by leiomyosarcoma, histiocytoma, and rhabdomyosarcoma [1–4]. The incidence is similar among Caucasians and African Americans with 0.3 to 0.2 cases per million, respectively [4].
Liposarcomas are soft tissue malignancies that present with a peak incidence of 70 years of age [4]. This tumor mostly occurs in the retroperitoneum, and less commonly in the lower extremities or paratesticular region [2]. Liposarcomas are classified into atypical lipomatous, tumor/well-differentiated liposarcoma, dedifferentiated liposarcoma, myxoid liposarcoma, pleomorphic liposarcoma, and mixed-type liposarcoma [5].
Tumor diameters greater than 10 cm are considered giant [2], and due to their appearance just inferior to the superficial inguinal ring, they present as a large scrotal mass [3]. Often, these tumors are mistakenly diagnosed as a hydrocele, cyst, hernia, hematocele, or lipoma. Dedifferentiated liposarcomas (DDLPS) tend to have a higher local recurrence rate, a greater potential to metastasize to distant sites, and a higher risk of death compared to well-differentiated low-grade liposarcomas (WDLPS).
On ultrasound, paratesticular liposarcomas appear as heterogeneous, solid, and hypoechoic lesions [2]. Any painless echogenic mass with heterogeneous architecture on ultrasound and relatively low vascularity should arouse suspicion of a liposarcoma [3]. CT and MRI are more specific and can differentiate areas of fatty components from soft tissue components. Histopathology, immunohistochemistry, and cytomorphological features also play a diagnostic role in these malignancies [2, 3].
Even though an scrotal incision was decided in our case, radical orchiectomy with wide local excision and high ligation of the spermatic cord with multimodality treatment, and long-term follow up are suggested [1, 2, 6, 7]. Although there has been no clear consensus on systemic therapy, research suggests that systemic therapy in conjunction with surgery in a selection of patients with DDLPS may reduce the risk of recurrence [6, 7]. Reasons to consider adjuvant radiotherapy would be for high-grade tumors or tumors with a high risk of local recurrence, since some of these tumors are radiosensitive. Chemotherapy could be used as another strategy in cases of dedifferentiated or metastatic disease [4–6, 8, 9] as in our case.
DDLPS are characterized by a highly amplified chromosomal region 12q13–15, including near-universal amplification of MDM2, a negative regulator of p53. Over 90% of DDLPS also express amplified amounts of the cell cycle regulator CDK4 [6]. Established prognostic factors for DDLPS include the degree of tumor resection, the tumor grade, staging, and the presence of metastases [6]. Overall survival reported at 1, 3, and 5 years was 91.3%, 64.0%, and 47.7%, respectively, with a median overall survival of 55 months [9]. Retrospective analyses of DDLPS patients have shown that MDM2 amplification is associated with decreased time to recurrence and worse disease-free survival [6]. Future directions in systemic treatment management may focus on newer therapy options to target the highly amplified expressions of MDM2 and CDK4.
CONCLUSION
Liposarcomas of the scrotum are rare. Diagnosis can be challenging, but careful history, examination, and imaging can aid in the diagnosis. The primary treatment modality, radical orchiectomy with wide local excision and high ligation, remains the best treatment strategy for overall survival. Multimodality therapy, along with long-term follow up, is suggested. Adjuvant chemotherapy offered on a case by case basis.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



