-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Catarci, Andrea Balla, Luigi Coppola, Unusual presentation of small bowel GIST: diffuse omental & mesenteric sarcomatosis, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa341, https://doi.org/10.1093/jscr/rjaa341
Close - Share Icon Share
Abstract
Gastrointestinal stromal tumors (GISTs) represent ~1% of gastrointestinal (GI) tract neoplasms. Unusual presentation of a small bowel GIST with diffuse omental and mesenteric implants in a symptomatic patient is reported. CT scan in a 68-years-old woman showed multiple processes with solid density and colliquation areas in the abdominal cavity. At surgery, an uncommon finding of multiple omental and mesenteric secondary implants was evident. The index mass with 40 cm of adjacent small bowel, omentum and all peritoneal lesions were completely removed. Definitive pathology report showed a small bowel GIST with focal areas of necrosis and high mitotic activity (35 mitosis/50 High Power Fields), with multiple metastases on mesentery and omentum. Patient was therefore submitted to adjuvant treatment with Imatinib and a close follow-up program. Small bowel GIST with high mitotic activity may present with diffuse omental and mesenteric peritoneal seedings. Complete surgical clearance remains the mainstay of treatment.
INTRODUCTION
Gastrointestinal stromal tumors (GISTs) represent the most common mesenchymal neoplasm of the gastrointestinal (GI) tract [1, 2]. Common GISTs locations are the stomach (50–60%) followed by the small bowel (20–30%) and the esophagus, colon and rectum [3]. GISTs can have atypical location in the omentum or in other sites as mesentery, retroperitoneum, gallbladder, abdominal wall, peri vesical tissue, pharynx, mediastinum, liver and pancreas [4], being defined as extra-gastrointestinal stromal tumors (EGISTs).
Being asymptomatic in 70% of cases, GISTs are often an incidental finding [2]. Nonspecific symptoms as well as nausea, vomiting, abdominal discomfort, weight loss or more severe conditions such as bleeding (haematemesis, melena or anemia), rupture of the tumor (acute abdomen), dysphagia and bowel or biliary obstruction are also described [2].
GISTs tumor cells express the tyrosine kinase KIT receptor (CD117), because of a mutation in the proto-oncogene c-kit, or another tyrosine kinase receptor such as the platelet-derived growth factor receptor alpha oncogene (PDGFRA) [1–3].
We report an unusual presentation of a small bowel GIST with diffuse omental and mesenteric implants in a symptomatic patient treated by surgical complete removal of the mass, omentum and metastases.
CASE REPORT
A 68-years-old woman (body mass index 35.6) was admitted with recent onset sideropenic anemia and not heart-related angina pectoris. At physical examination, a slightly mobile mass in the left iliac fossa was palpable. Carcinoembryonic antigen, carbohydrate antigen 19.9 (CA 19.9) carcinoma antigen 125 (CA 125) were normal. Upper and lower GI endoscopy were negative for any lesion. The patient was submitted for computed tomography (CT) scan, showing multiple solid density processes with colliquation areas in the abdominal cavity (Fig. 1). The greatest mass (8.0 × 5.6 cm) was located in the pelvis in close contact with the uterine fundus and the small bowel (Fig. 2). Cranially to this process, at least 20 other tumors with a diameter between 0.8 and 3.8 cm could be appreciated.
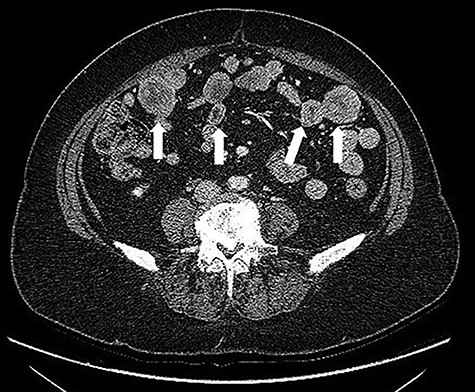
CT scan: multiple solid density processes with colliquation areas within them (white arrows) in the abdominal cavity.
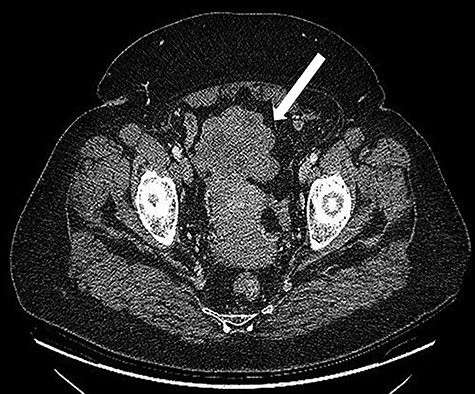
CT scan: Bulky (8.0 × 6.5 cm) mass located in the pelvis in close contact with the uterine fundus.
After multidisciplinary team discussion, patient was submitted to diagnostic laparoscopy. Intra-operatively, a friable and easily bleeding bulky pelvic mass was found (~5 × 8 cm), originating from the free margin of an ileal loop without infiltration of uterus and ovaries. Multiple secondary omental and mesenteric lesions were confirmed. A midline laparotomy was therefore performed, showing multiple omental and mesenteric secondary implants (Fig. 3) and a bulky pelvic mass originating from the small bowel (Fig. 4). Omentectomy, small bowel resection and complete removal of all mesenteric implants were performed. Postoperative course was uneventful with discharge on postoperative Day 6.
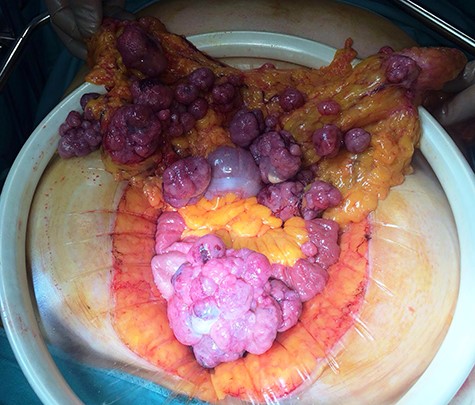
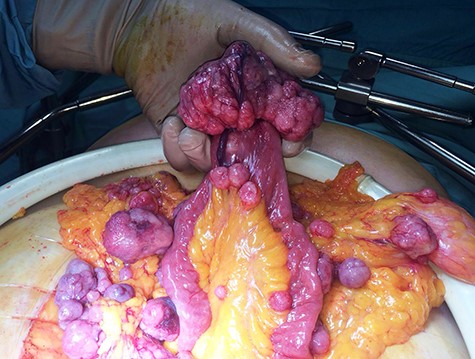
Definitive pathology, according to Miettinen [4], showed a small bowel GIST with focal areas of necrosis and high mitotic activity (35 mitosis/50 HPF). Immunohistochemistry was positive for CD117, DOG1, CD34 and negative for S100 and smooth muscle actin. Ten metastases on the small bowel mesentery and various others on the omentum were also reported. After oncological counseling, patient was submitted to adjuvant treatment with Imatinib mesylate 800 mg twice daily of and a close follow-up program, remaining disease-free after 24 months.
DISCUSSION
GISTs are common mesenchymal neoplasms with a predominance in elderly patients (median age 66–69 years), such as in this report [2]. Familial or sporadic GISTs are both described but often no differences are observed in phenotypic and molecular features [2]. In case of small lesions, GISTs appear at contrast enhanced CT scan as a solid soft mass with heterogeneous enhancement; larger tumors and/or large exophytic grows may be due more difficult to diagnose due to necrosis, hemorrhage and degenerating elements. Small bowel GISTs may present as an incidental finding on abdominal imaging, but are often large at diagnosis, and they more commonly present with ulceration and bleeding (occult or massive). Other common presenting symptoms include pain, weight loss, perforation or a palpable mass. Because they tend to enlarge extraluminally, obstruction is rare and typically a late presenting symptom [1, 2]. The finding of multiple abdominal lesions on CT scan performed for unexplained anemia, such in the present case, is rather uncommon and may well be misleading [5]. A consistent series of omental GISTs identified multiple masses in ~40% of cases, suggesting a possible metastatic origin [6]. Actually, omental and mesenteric EGISTs are generally derived from stomach and small bowel, respectively, representing neoplastic growths that during their development detached from their GI origin.
When it is possible to perform a radical clearance of the tumor(s), surgery is still the gold standard treatment of GISTs and complete resection of the lesions entails better prognosis [2]. To avoid mass rupture, which entails a higher risk of recurrence, laparoscopy is recommended for lesions ~5 cm in diameter [7, 8]. From 2001, after the discovery of the KIT and PDGFRA mutations, it is possible to use a tyrosine kinase inhibitor (Imatinib mesylate) to treat GISTs in neoadjuvant or adjuvant setting with a median increase in survival ~60 months [7].
Independently from the treatment, based on tumor size, mitotic index and localization of GISTs, Miettinen et al. [9] suggested a classification for the risk of progressive disease, with peritoneal tumors showing the lowest survival rate [10]. Based on these figures, adjuvant therapy with Imatinib mesylate was suggested in the present case due to a very high risk to develop progressive disease (≅85%).
Anyway, even in case of a more favorable prognosis, the long-term follow-up of GISTs is uncertain due to an unpredictable behavior, and patients should be submitted to a close follow-up program by periodic CT scan, particularly in the first 3–5 years, due to the high probability of disease recurrence during this period.
CONCLUSIONS
GIST is often considered a low malignancy tumor due to the asymptomatic course of the disease in the majority of cases. Anyway, delay in diagnosis can lead to clinical presentation when the tumor is already metastatic. In case of omental and mesenteric spread, whenever possible, surgery remains the best first line of treatment. Alternatively, imatinib mesylate can be employed in a neo-adjuvant setting to reduce the tumor load before surgery.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
- computed tomography
- immunologic adjuvants
- pharmaceutical adjuvants
- follow-up
- intestine, small
- mesentery
- mitosis
- necrosis
- neoplasm metastasis
- omentum
- surgical procedures, operative
- neoplasms
- pathology
- peritoneum
- imatinib mesylate
- gastrointestinal stromal tumor
- Abdominal cavity
- liquefactive necrosis
- sarcomatosis
- implants
- surgical clearance



