-
PDF
- Split View
-
Views
-
Cite
Cite
Jonathan Sivakumar, Gregor Brown, Laurence Galea, Julian Choi, An intraoperative diagnosis of sclerosing encapsulating peritonitis: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa329, https://doi.org/10.1093/jscr/rjaa329
Close - Share Icon Share
Abstract
Primary sclerosing encapsulating peritonitis (SEP) is an idiopathic and rare condition characterized by chronic peritoneal inflammation. We describe the case of an intraoperative diagnosis of SEP, presenting as a mimicker of small bowel obstruction. The patient was a 59-year-old male with suspected small bowel obstruction. On exploratory laparotomy, it was noted that there was thick fibrous tissue involving the visceral and parietal peritoneum enveloping grossly dilated loops of small bowel. This case reports on the histopathological features of peritoneal biopsies as well as radiological findings. There is no consensus regarding the standard management for idiopathic SEP. The present case demonstrates a significant improvement in the patient’s condition with conservative management alone. A critical teaching point is that in the absence of an obvious cause, SEP is a rare but important differential diagnosis for surgeons to consider in the context of recurrent bowel obstruction.
INTRODUCTION
Primary sclerosing encapsulating peritonitis (SEP) is a rare condition characterized by chronic peritoneal inflammation and is typically of unknown aetiology [1]. Few cases have been described in the literature, although it clinically manifests as a recurrent small bowel obstruction. The morphological description of SEP in previous reports is that of encasement of distended bowel loops within a ‘cocoon-like’ thick fibrocollagenous peritoneal membrane [2]. We describe the case of an intraoperative diagnosis of SEP with accompanying operative, histopathological and radiological images.
CASE REPORT
The 59-year-old male presented to hospital complaining of severe nausea and generalized abdominal pain requiring admission. The patient had an episode of gastroenteritis 6 month prior, and since then experienced multiple episodes of colicky abdominal pain with intermittent vomiting. The patient had no other medical history of significance. On physical examination, he was hemodynamically stable, although generally tender with a distended abdomen. His laboratory results and chest X-ray were unremarkable. A computed tomography scan was suggestive of a subacute small bowel obstruction with a high
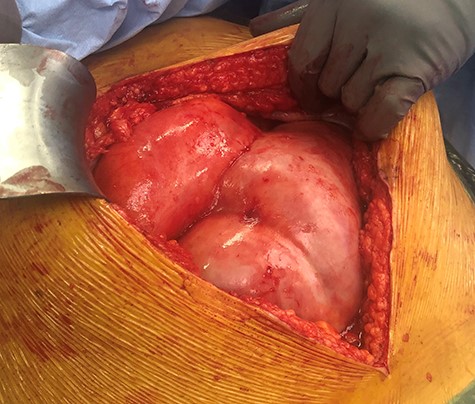
Operative appearance of grossly dilated bowel and thickened peritoneal membrane.
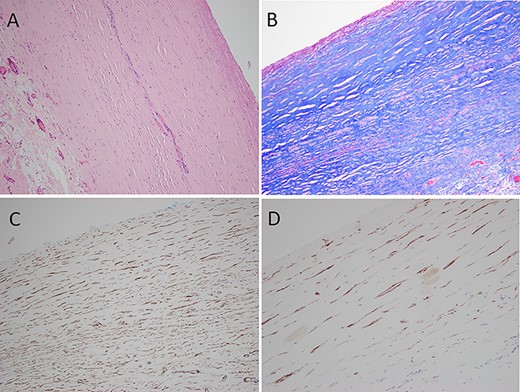
Histopathological examination (A) thick dense collagenous fibrous tissue with denuded mesothelial lining (H&E), (B) Masson trichrome stain confirming collagenous fibrous tissue, (C) smooth muscle actin immunohistochemistry showing myofibroblasts within the fibrous tissue, (D) D2-40 immunohistochemistry showing lymphatic endothelial cells.
suspicion of an internal hernia through an omental defect. Given the patient’s clinical status correlated with these radiological findings, he was counselled for surgery and underwent an exploratory laparotomy.
Intraoperatively, thick fibrous tissue involving the visceral and parietal peritoneum was found enveloping grossly dilated loops of small bowel (Fig. 1). There was no evidence of a transition point contributing to this patient’s obstruction. As any manipulation or mobilization of the bowel was thought to be high-risk of causing enterotomy, a decision was made to close the abdomen after collecting peritoneal washings and biopsies for histopathology and microbiology.
The patient was monitored on the ward post-operatively during which he made a good recovery with broad-spectrum antibiotics and supportive management. He was discharged home 9 days following his operation, after demonstrating significant improvement in gastrointestinal function, with reduced pain and able to tolerate oral intake.
Intraoperative peritoneal swabs tested negative for acid-fast bacilli and no organisms were cultured from peritoneal fluid. Haematoxylin and eosin stained sections of peritoneal biopsy showed markedly thickened collagenous fibrous tissue with adherent loose fibrovascular connective tissue and intervening myofibroblast-like cell, the latter supported by smooth muscle actin immunohistochemistry and a Masson trichrome stain (Fig. 2). There was an associated mild focal chronic inflammatory cell infiltrate including lymphocytes and histiocytes. The mesothelial lining was completely denuded. D2-40 immunohistochemistry showed lymphatic endothelial cells within collagenous fibrous tissue. There was no evidence of malignancy or granulomatous inflammation. Post-operative blood tests found that beta-2 microglobulin, lactate dehydrogenase, immunoglobulin G protein studies, carcinoembryonic antigen and carbohydrate antigen 19–9 were all within normal ranges.
On retrospective review of the case at a multidisciplinary team meeting, the original scans were reviewed and demonstrated central congregation of the small bowel loops encased within a ‘cocoon-like’ thick peritoneal membrane (Fig. 3), characteristic of SEP [3]. A consensus was reached whereby this patient’s presentation was attributed to SEP, otherwise termed ‘abdominal cocoon syndrome’. This diagnosis is exceedingly difficult to make preoperatively, and previous reports have only identified this condition on exploratory laparotomy [4, 5]. Furthermore, given its rare incidence, as well as non-specific clinical and radiological findings, SEP is easily overlooked [6]. This diagnosis was also later confirmed on intestinal ultrasound which demonstrated a centrally clumped ‘cauliflower-like’ appearance of the small bowel, encased within a thickened capsule, depicting a trilaminar sign.
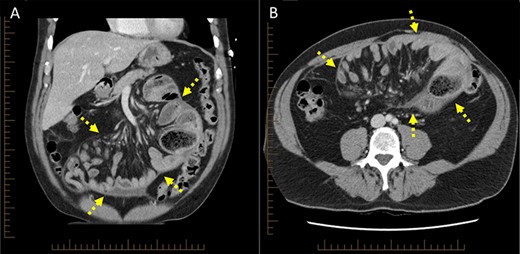
Computed tomography scan (A) coronal cross-section, (B) axial cross-section demonstrating ‘cocoon-like’ membrane enveloping dilated loops of small bowel (arrow).
DISCUSSION
Although existing cases in the literature have predominantly been idiopathic, other secondary causes that have been associated include peritoneal dialysis, disseminated tuberculosis, sarcoidosis, gastrointestinal malignancy and the use of povidone-iodine for abdominal washout [7]. An underlying secondary cause of this patient’s presentation was investigated but not identified.
There is no consensus regarding the standard management approach for idiopathic SEP. Case reports have focused on surgical treatments including peritoneal sheath excision and adhesiolysis [5]. Resection of the bowel is typically only pursued when the appearance is ischaemic and non-viable. Some authors have also advised against a primary anastomosis in this setting due to the high rate of anastomotic leak and associated morbidity [8]. Furthermore, an aggressive surgical approach may lead to an increased risk of intraoperative enterotomy and subsequent enterocutaneous fistula formation. Medical treatments such as tamoxifen, corticosteroids and other immunosuppressive agents have been reported in the management of SEP [9]. We report a significant improvement in the patient’s condition with conservative management, consisting of bowel rest and nutritional support. This patient was also commenced on oral prednisolone and he will continue to have close clinical follow-up with a focused small bowel ultrasound. In the absence of an obvious cause, SEP is a rare but important differential diagnosis for surgeons to consider in the context of recurrent bowel obstruction.
ETHICS APPROVAL
This case report was approved by the ethics committee of Research Development and Governance Unit at Epworth Healthcare.
INFORMED CONSENT
Written informed consent was obtained from the patient to publish this case report.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



