-
PDF
- Split View
-
Views
-
Cite
Cite
Raghav Chandra, Epameinondas Dogeas, Nicole Nevarez, Mathew Augustine, Sergio Huerta, Peritonitis from perforated sigmoid mass as the first manifestation of metastatic squamous cell lung cancer: a case report and review of literature, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa315, https://doi.org/10.1093/jscr/rjaa315
Close - Share Icon Share
Abstract
Lung cancer (LC) is an aggressive malignancy with early metastatic spread and poor prognosis. Gastrointestinal metastases from primary LC are extremely rare with highly variable presentations. In this report, we review the case of a patient who presented with peritonitis secondary to perforated sigmoid mass as the first manifestation of metastatic squamous cell LC.
INTRODUCTION
Squamous cell non-small cell lung cancer (SC-NSCLC) is an aggressive tumor and the second most common histologic type of lung cancer (LC). LC is the most common cause of cancer-related mortality worldwide [1]. Patients with LC suffer from early metastasis, most often to the liver, lungs, bone and brain [2]. Symptomatic metastasis to the gastrointestinal tract from LC is extremely rare. In this report, we discuss a 69-year-old male who presented with peritonitis secondary to sigmoid perforation due to intestinal metastasis from primary SC-NSCLC as the first manifestation of LC.
CASE REPORT
The patient is a 69-year-old white male with a history of diabetes mellitus, hypertension and 56 pack-year smoking who presented to our emergency department with abdominal distension and pain. Physical examination showed severe, diffuse abdominal tenderness with rebound and guarding consistent with peritonitis. Laboratory studies revealed a white blood cell count: 9.4 × 109 cells/L, hemoglobin: 10.9 g/dL, platelets: 370 × 109 cells/L and creatinine: 1.29 mg/dL. Computerized tomography (CT) imaging of abdomen/pelvis demonstrated thickening of the sigmoid colon, pneumoperitoneum, intraperitoneal free fluid, multifocal liver lesions, and peritoneal and omental implants concerning for metastatic perforated sigmoid carcinoma (Fig. 1). The patient was taken emergently to the operating room for an exploratory laparotomy. A perforated sigmoid and multiple liver and omental lesions were encountered. A sigmoidectomy with end colostomy, partial omentectomy and wedge resection of liver segment III were performed. The patient was admitted to the surgical intensive care unit where he received routine postoperative care.
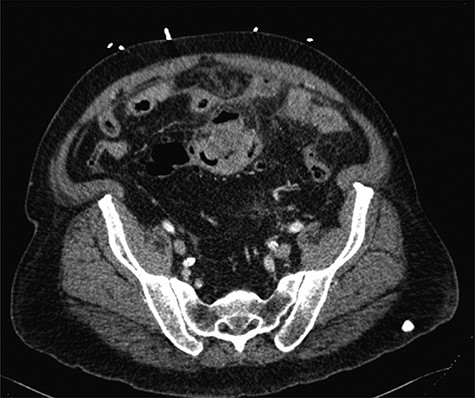
Axial CT imaging of the abdomen, which demonstrates focal thickening of the sigmoid colon with surrounding free fluid.
Pathologic analysis revealed that the resected specimens were consistent with poorly differentiated SC carcinoma with immunohistochemical staining reflecting a primary pulmonary source. Tumor cells stained positive for CK5/6 and p40 and were negative for CK7, CK20, villin and TTF1. This established the diagnosis of intra-abdominal metastasis from SC-NSCLC. A radiograph and subsequent CT imaging of the chest confirmed the presence of a right perihilar mass obstructing the right upper and middle lobe bronchi, encasement of the right pulmonary artery branches and extensive bulky mediastinal lymphadenopathy, consistent with primary LC (Fig. 2).
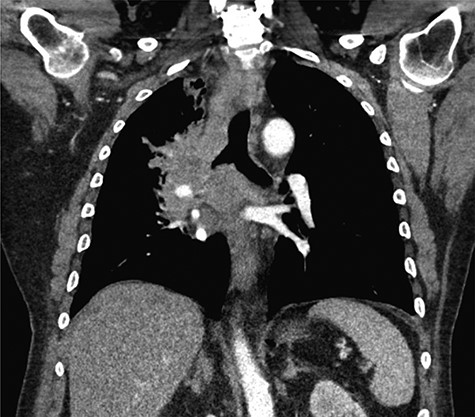
Coronal CT imaging of the thorax demonstrating a large, heterogeneous right perihilar mass with invasion of multiple segmental branches of the right pulmonary artery.
DISCUSSION
We present an unusual case of peritonitis secondary to a perforated sigmoid mass found to have metastatic right lung SC-NSCLC.
LC is responsible for the greatest number of cancer deaths worldwide [1]. The most common subtype of LC is adenocarcinoma, followed by SC, small cell and others [3]. The only cure for localized SC-NSCLC is surgical resection and adjuvant chemotherapy as well as radiation therapy as necessary. Unfortunately, 40% of patients present with Stage IV disease [3]. Localized LC has a 5-year survival of 57.4%, whereas metastatic disease has a dismal 5-year survival of 5.2% [4].
Symptomatic gastrointestinal metastases (GM) from primary LC are rare, but maybe encountered in ~12% of patients on autopsy [5]. The pathophysiology of GM from LC is poorly elucidated but is likely from hematogenous spread [6]. In an autopsy analysis of 470 patients with GM from LC, the most common site of GM was the small bowel [5]. The most commonly associated LC subtype is controversial, with some series highlighting squamous cell, whereas others report large cell carcinoma [6, 7].
Symptoms of GM from LC can range from mild abdominal discomfort, gastrointestinal bleeding, and intussusception to peritonitis from perforated viscus [5–7]. Diagnosis requires a high index of suspicion. In patients with known LC presenting with abdominal symptoms, cross-sectional imaging is crucial for ascertaining presence of carcinomatosis or bowel metastases. Positive fecal occult blood testing should be followed up with colonoscopy and raise concern for GM. It is important to differentiate between metastasis from LC and two distinct tumors (i.e. lung and colon cancer)—as the prognosis and treatment algorithms are markedly different. Thus, it is imperative to obtain a tissue biopsy of the mass for immunohistochemical analysis.
Positive tumor markers for colon adenocarcinoma include CK20, AMACR, CDX-2, villin and MUC2 [8]. Markers reflective of SC-NSCLC include p63, CK5/6 and p40 [9]. The resected specimen from our case was positive for CK5/6 and p40 and negative for villin and CK20, confirming the diagnosis of SC-NSCLC as opposed to colon adenocarcinoma.
The treatment of GM is not standardized given the limited number of reported cases and the wide variety of clinical presentations. Patients with incidentally found GM may undergo palliative chemotherapy or radiation. In cases of impending bowel obstruction, perforation or bleeding, segmental bowel resection might be indicated to prevent these immediately fatal complications. Acute obstruction or perforation may require emergent laparotomy. In planned cases, primary anastomosis may be feasible, but in patients with abdominal sepsis from perforation, end ileostomy or colostomy may be necessary.
GM from LC has a poor prognosis with a median duration of survival from diagnosis of 100 days [7]. Survival as high as 13 months has been documented, particularly with early surgical palliation [10]. In a case series of nine patients with acute complications from LC GM requiring emergent laparotomy, four out of eight patients (50%) who survived the initial resection were alive at 6 months [10]. Thus, there is a role for surgical palliation in these patients. In this case, our patient did not die as a result of sigmoid perforation, but ultimately from obstructive bronchopneumonia.
Gastrointestinal metastasis from LC is extremely rare, but is likely under-recognized. In this case report, we present a case of a 69-year-old male who presented with peritonitis from a perforated sigmoid mass found to have metastatic SC lung carcinoma. This case highlights the need for a high index of suspicion for GM in patients with LC who present with abdominal symptoms and demonstrates that acute abdominal symptoms may be the first manifestation of metastatic LC. The best way to distinguish between primary gastrointestinal malignancy and metastatic LC is on immunohistochemistry. The treatment of GM is highly dependent on the severity of disease and patients with acute perforation often require emergent surgical intervention. Although the prognosis of GM from LC is overall poor, there is evidence to suggest a role for surgical palliation for these critically ill patients.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



