-
PDF
- Split View
-
Views
-
Cite
Cite
Mekhola Hoff, Philippa Leighton, Sarah A Hosgood, Michael L Nicholson, Anastomosis of dual renal transplant veins, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa310, https://doi.org/10.1093/jscr/rjaa310
Close - Share Icon Share
Abstract
As there is usually considerable overlap in the renal venous drainage, it is often possible to ligate supernumerary transplant renal veins in order to simplify the implantation procedure. Nonetheless, decisions about whether to implant multiple veins can be difficult and are usually made subjectively. Here, we describe the use of intraoperative Doppler ultrasound as an adjunct to decision-making when there are two renal veins and a novel technique for the sequential anastomosis of dual veins. The kidney was reperfused after anastomosis of the main renal vein with the second vein clamped. On-table Doppler ultrasound demonstrated reversed flow in diastole indicating that the second renal vein also needed to be anastomosed. By clamping the external iliac vein inferior to the first venous anastomosis it was possible to complete the lower polar renal vein anastomosis to the external iliac vein without interrupting the perfusion of the kidney.
INTRODUCTION
Renal arteries are end arteries, so during kidney transplantation it is important to preserve all but very small multiple arteries. In contrast, the major intra-renal veins are usually characterized by an extensive system of anastomotic communications. The clinical correlate of this is that it is usually possible to ligate smaller renal transplant veins without compromising the venous drainage of the kidney.
Kidneys with dual veins that are each substantial or even equal in size are more difficult to deal with as both veins may require anastomosis to achieve unimpeded venous outflow. Here, we describe a technique for deciding whether a supernumerary vein requires anastomosis and a new technique for performing renal venous anastomoses sequentially.
CASE REPORT
We were provided with a right kidney from a 55-year-old male donation after brain death donor who had died as a result of an intracerebral haemorrhage. The kidney had a single renal artery with aortic patch and a single ureter but there were dual renal veins. The main hilar renal vein had a diameter of 12 mm, the lower polar vein had a diameter of 8 mm and the veins were separated by a distance of 4 cm. The veins had been divided at the level of the inferior vena cava; a caval tube had not been provided.
The recipient was a 47-year-old female with a body mass index of 28.5 m2/kg and end-stage renal failure secondary to chronic pyelonephritis. The kidney was implanted into the right iliac fossa. The main hilar renal vein was anastomosed end to side to the external iliac vein using 5/0 polypropylene and the renal arterial patch was anastomosed end to side to the external iliac artery using 5/0 polypropylene. The lower polar vein was controlled with a fine atraumatic vascular clamp and not anastomosed initially. On release of the arterial and venous clamps, the kidney appeared globally well perfused and some urine production was observed. Nonetheless, both the main hilar and lower polar veins were distended and felt congested. On-table Doppler ultrasound was performed by members of the surgical team. This confirmed that the kidney was globally perfused but the waveforms showed reversal of flow in diastole throughout the kidney. The external iliac vein was clamped inferior to the upper renal vein anastomosis and at the inguinal ligament and the lower polar vein was then anastomosed end to side to the external iliac vein using 5/0 prolene (Fig. 1). On clamp release the kidney’s appearance improved immediately and both renal veins became soft and easily compressible. A repeat intraoperative Doppler ultrasound examination demonstrated excellent perfusion of the whole kidney and a triphasic waveform with forward flow in diastole. These findings were confirmed in the recovery room by an experienced radiologist. The cold ischaemic time was 12 h 18 min and the anastomosis time was initially 32 min with a further 10 min for the lower polar venous anastomosis. The kidney had initial function and at 12 month follow-up the recipient’s serum creatinine was 103 μmol/l.
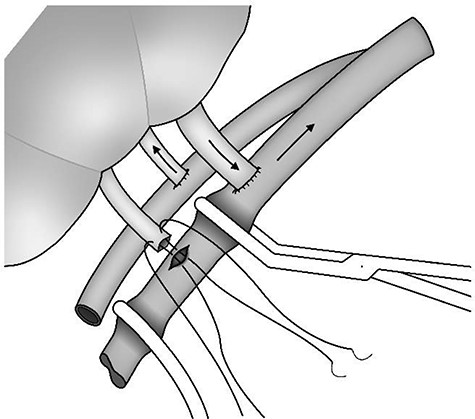
Sequential anastomosis of a lower polar renal vein while the kidney is being perfused.
DISCUSSION
The prevalence of multiple renal veins has been investigated in anatomical studies and by CT angiography [1–5]. In a meta-analysis of 105 studies, the mean incidence of multiple renal veins was 16.7% [1]. Multiple renal veins were much more common on the right (16.5%) than on the left side (2.1%). In one study multiple right-sided veins were found in 31% of cases [2]. In consequence, transplant surgeons need to make decisions about how to deal additional renal veins on a frequent basis [3].
Where dual renal veins are present and the second vein is substantial, it has been our practice to anastomose the larger vein first, followed by the artery and then to reperfuse the kidney with the second vein controlled with an atraumatic vascular clamp. In our experience this provides sufficient venous drainage in the vast majority of cases. The criteria for adequate venous drainage are good macroscopic perfusion of the kidney, a lack of tension in the renal parenchyma and a soft compressible renal vein post-anastomosis. Finally, the clamped smaller renal vein should not be distended and congested after the main renal vein is in circuit. If all of these criteria are met, then the smaller vein can be sacrificed by suture-ligation at the renal hilum. This considerably simplifies and shortens the transplant operation.
If it is not entirely clear, using these subjective criteria, that the venous drainage is adequate without anastomosing the second renal vein we have shown here that intraoperative Doppler ultrasound scanning can be used to provide a more objective assessment. Direct application of the ultrasound probe to the surface of the kidney intraoperatively simplifies the techniques of ultrasound scanning considerably. In this case the initial ultrasound assessments were made by the surgical team. With the help of their radiological colleagues, surgeons can quickly learn the basic ultrasound techniques required to acquire and assess Doppler waveforms intraoperatively. We would encourage transplant surgeons to add Doppler ultrasound examination to their skill set so that quick decisions can be made without recourse to busy radiological colleagues.
The surgical technique described here requires intraoperative planning of the siting of the main renal venous anastomosis. This should provide sufficient external iliac vein distally for a second anastomosis should this become necessary. Careful positioning of the vascular clamps for the second venous anastomosis is also important. This allows the second venous anastomosis to be completed while the kidney is being perfused, thus reducing the warm ischaemic interval. This plan is only suitable for dual renal veins when the larger hilar vein is in a more superior anatomical position than the lower vein.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.



