-
PDF
- Split View
-
Views
-
Cite
Cite
Miguel Johnson, Lorna Cook, Fabio I Rapisarda, Dibendu Betal, Riccardo Bonomi, Oncoplastic breast surgery technique for retroareolar breast cancer: a technical modification of the Grisotti flap in patients with short nipple to inferior mammary crease distance, Journal of Surgical Case Reports, Volume 2020, Issue 9, September 2020, rjaa285, https://doi.org/10.1093/jscr/rjaa285
Close - Share Icon Share
ABSTRACT
The introduction of breast conservation surgery together with advances in oncoplastic techniques has revolutionized the management of retroareolar breast tumours. Traditionally, cancers in this location were often managed with central excision and primary closure or mastectomy. More recently, oncoplastic breast-conserving techniques such as the Grisotti mammoplasty have been increasingly encouraged as an alternative option as it allows oncological safe margin resections while restoring cosmesis. The use of a Grisotti flap enables safe resection of a retroareolar tumour with concurrent reconstruction of the defect using a local rotational advancement dermoglandular flap allowing a satisfactory cosmetic result in term of contour and projection. This technique is often limited to those patients with sufficient native nipple-inferior mammary fold (IMF) distance to accommodate for some inevitable post-operative reduction in this distance. We describe a modification of the original description, such that satisfactory cosmetic outcome can be achieved, even in patients with a short nipple areolar complex to inframammary fold distance.
INTRODUCTION
The surgical management of tumours in the retroareolar region of the breast continues to remain a challenge with many surgeons opting for central excision and primary closure or complete mastectomy to achieve clearance. However, there is a trend towards providing less mutilating procedures while improving cosmesis.
More recently, the advent of COVID-19 virus has led to significant disruption in regular protocols, standard of care and management pathways for patients in the UK and worldwide [1]. This has led to more innovations as surgeons are tasked with providing the best care for their patients, which involves less mutilating procedures where clinically possible.
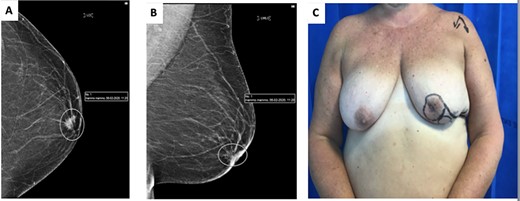
(A) cranio-caudal view (CC) mammogram showing speculated retroareolar mass. (B) Latero-medial oblique (LMO) view mammogram showing speculated retroareolar mass. (C) Preoperative image of left breast, note retracted nipple.
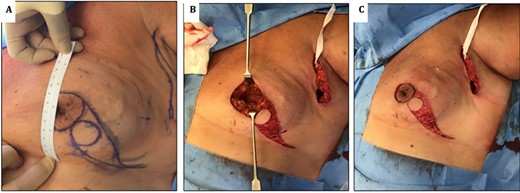
(A) images showing preoperative markings of modified Grisotti flap. Note the short nipple to IMF crease length, which would significantly shorten further if a standard Grisotti technique were undertaken. (B) En bloc resection of NAC and tumour creating a significant defect. (C) Harvesting of modified Grisotti flap with de-epithelialization of the skin surrounding the flap with preservation of the medial triangle skin (delimitated superiorly by the areola and inferiorly by disc of skin to be rotated).
The standard Grisotti flap provides an excellent oncoplastic option for patients with retroareolar cancers and moderate size breast. It also allows the preservation of the breast while achieving a satisfactory cosmetic result in terms of contour and projection [2]. However, the standard Grisotti flap is not ideal for patients with a short nipple areolar complex (NAC) to inferior mammary fold (IMF). We describe a technical modification that provides satisfactory aesthetic results even in patients with a relatively short NAC to IMF as exemplified in our case below.
CASE REPORT
A 62-year-old woman underwent routine screening bilateral mammogram which revealed the presence of a spiculated density behind the left NAC (Fig. 1A and B). On clinical examination the left nipple appeared retracted and indurated (Fig. 1C). An ultrasound assessment detected a 12-mm irregular mass corresponding to the mammographic abnormality with an abnormal lymph node in the ipsilateral axilla. Core biopsies confirmed the presence of a grade 2 invasive ductal carcinoma ER8 PR8 Her2 negative, metastasized to the axilla. Staging CT scan confirmed no evidence of distant metastatic disease. MDT decision was made to proceed to offer mastectomy or central excision with either primary closure or local flap reconstruction and axillary clearance. Following informed consent, a central quadrantectomy and a Grisotti flap were undertaken.
The NAC–IMF distance in this patient was measured pre-operatively as 6 cm (Fig. 2A). Since the traditional flap markings (Fig. 4B) (use of a 4 cm disc of skin to form the neo-areola, based at the 6 o’clock position) would have resulted in an unacceptable shortening of the NAC–IMF distance, the technique was modified as described below.
Technique
Markings were performed preoperatively. The diameter of the skin disc used to form the neo-areola was reduced to a 3 cm instead of the usual 4 cm and was positioned in the lower-outer quadrant of the breast rather than at the standard 6 o’clock position (Fig. 2A).
Following en bloc excision of NAC and the tumour (Fig. 2B), the medial triangle of the skin (delimitated superiorly by the areola and inferiorly by the disc of skin to be rotated) was preserved to avoid creating a ‘teardrop’ shape of the new areola at the time of closure as described by Della Rovere et al. [3] (Fig. 2C).
As per standard procedure [2], the undermining of the lateral remaining glandular tissue was then performed to facilitate the advancement, rotation and medialization of the glandular tissue in order to increase the projection of the breast (Fig. 3A and B). The patient had good aesthetic results and maintenance of an acceptable neo-nipple to IMF distance of 6.5 cm (Fig. 4).
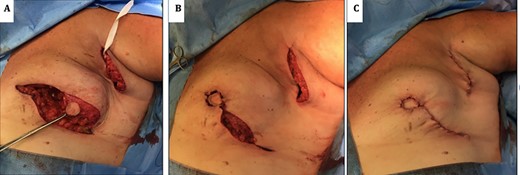
(A) image showing standard undermining of the lateral aspect remaining glandular tissue to facilitate advancement and rotation into central defect. (B) Insetting of modified Grisotti flap. (C) Closure of donor site and completion insetting of flap.
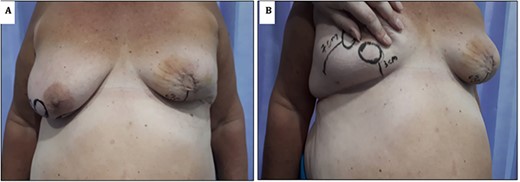
(A) Image showing 1-week post-operative results; note the maintenance of neo-nipple to IMF crease distance of 6 cm. (B) Contralateral breast showing location of standard Grisotti disc; note the relatively short NAC-IMF of 3 cm that would result if this technique were undertaken.
DISCUSSION
Tumours of the retroareolar region of the breast have traditionally been managed by mastectomy or central excision and primary closure. However, driven by an increasing emphasis on the importance of patient-reported satisfaction as an outcome measure, together with advances in oncoplastic techniques, practice is beginning to change. Surgeons are increasingly offering their patients breast-conserving surgery (BCS) in an attempt to reduce the mutilation and psychological morbidities associated with a mastectomy.
More recently close collaboration between oncological and plastic surgical teams has led to significant advancements in the field of BCS, in particular oncoplastic strategies. These techniques permit excision of larger volumes of the surrounding tissue around the cancer ensuring adequate oncological marginal clearance while equally allowing reconstruction of the resultant defect with excellent aesthetic results [4].
Several oncoplastic techniques are utilized, broadly classified into volume replacement or volume displacement techniques. Despite these advances, the management of retroareolar tumours remains challenging to achieve a satisfactory result as central excision and primary closure may produce poor cosmetic outcomes such as contour defects. Some surgeons instead opt to perform a mastectomy, particular skin-sparing mastectomy following by immediate breast reconstruction using autologous or implant-based techniques [5, 6].
Other approaches described for retroareolar tumours include an elliptical incision or a peri-areolar circular incision including the NAC and purse string closure of the defect [6]. These however may lead to flattening of the central aspect of the breast. The Grisotti flap has become a more acceptable standard approach as it allows reconstruction of the defect, following quandrantectomy, by utilizing an advancement and rotation of a random pattern dermoglandular pedicle [2, 7]. This technique can yield excellent cosmetic results and avoid mastectomy in selected patients [2, 8].
Grisotti’s original description [7] involved the creation of a circular disc of skin inferior to the NAC. This dermoglandular flap is then advanced and rotated into the defect. Based on this description, however, it can be inferred that this flap is not ideal for patients with a short NAC to IMF distance, as this flap will invariably shorten the NAC to IMF distance further. In our presented case, we propose a technical modification that allows adequate reconstruction, even in patients with a relatively short NAC to IMF distance (Fig. 4B). The inferolateral placement of the disc still allows adequate rotation and advancement of the circular disc of skin used to replace the areola, avoiding the shortening of the NAC to IMF distance and equally maintaining a natural projection and an aesthetically pleasing contour of the breast (Fig. 4).
There is a mounting evidence that performing a Grisotti flap for retroareolar tumours is oncologically safe in properly selected patients [2, 9, 10]. This modification of the Grisotti flap is a safe and offers a good alternative for patients with retroareolar tumours with a short NAC–IMF distance and allows expansion of the oncoplastic surgeon’s armamentarium.
FUNDING
None.



