-
PDF
- Split View
-
Views
-
Cite
Cite
Luke Geoghegan, Dariush Nikkhah, Ten technical considerations revisited in tissue expansion, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa273, https://doi.org/10.1093/jscr/rjaa273
Close - Share Icon Share
Abstract
Tissue expansion is a versatile tool in resurfacing soft tissue defects of the breast, extremity, head and neck that involves the placement of a temporary implant adjacent to a soft tissue defect. Incremental expansion exploits the viscoelastic properties of skin to generate a skin flap that can be used to resurface defects and provide cover over permanent protheses. Infection, implant extrusion and skin necrosis are recognized complications of tissue expansion. This article presents a revised framework of 10 technical factors to reduce the risk of complications and optimize outcomes with tissue expansion using an illustrative case presentation.
INTRODUCTION
Tissue expansion utilizes the viscoelastic properties of skin to provide autologous soft tissue coverage with skin that is of near-identical colour, texture and hair-bearing potential and minimal donor site morbidity. The use of tissue expansion has been reported in breast, extremity, head and neck reconstruction, although there is a higher risk of complications and failure when used in the extremities [1]. Tissue expansion may be complicated by infection, haematoma and seroma formation, flap necrosis due to excessive surface tension, wound dehiscence and implant erosion [2]. In their seminal paper published in 2005, Hudson et al. [3] outline 10 rules for reducing the risk of complications when using tissue expansion for burns reconstruction. Here, we present a refined list of 10 rules for optimizing outcomes following tissue expansion with an illustrative case report based on our experience.
CASE REPORT
We present the case of a 29-year-old woman who had undergone wide local excision for malignant melanoma affecting the vertex of her scalp, resulting in a 7 × 6 cm soft tissue defect initially reconstructed using a split-thickness skin graft, see Fig. 1. Two rectangular expanders were implanted lateral to the defect on the mid-scalp, see Fig. 2 with two closed suction drains left in the soft tissue pocket, see Fig. 3. Expansion was commenced 2 weeks postoperatively at regular intervals using an implanted valve port system over the course of 3 months to reach a total volume of 140 ml per implant. Expanders were removed with primary closure of the leading edges of each expanded skin flap. At 2-month follow-up, an excellent aesthetic result was obtained, see Fig. 4, without complication.
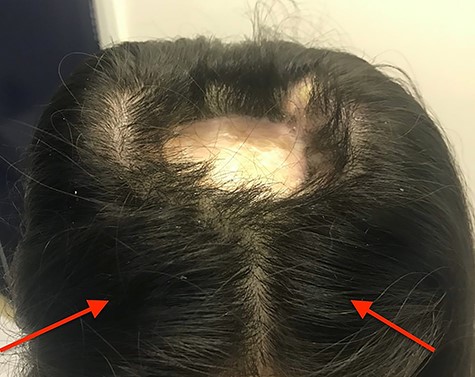
Clinical photograph taken after implantation of tissue expanders– a 7 × 6 cm ovoid soft tissue defect initially reconstructed using split thickness skin grafting can be seen at the mid-scalp; red arrows indicate port sites that were placed, anterior to the expander to avoid inadvertent damage during expansion, rectangular tissue expanders were sited laterally to the mid-scalp defect and inflated to a total volume of 140 ml before tissue expander removal.
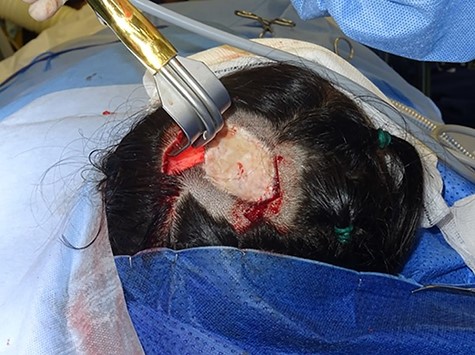
Intraoperative photograph taken during tissue expander insertion demonstrating dissection of the soft tissue pocket through a remote incision with the assistance of a lighted retractor.
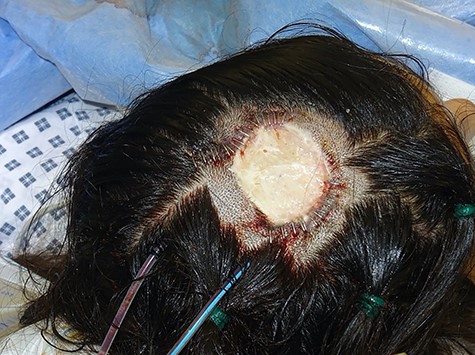
Intraoperative photograph taken following tissue expander insertion- rectangular expanders were inserted using a remote incision lateral to the midscalp defect; double-layered closure was used with the assistance of a malleable retractor for deep dermal closure followed by skin staples, closed system suction drains were sited adjacent to the leading edge of each expansion flap.
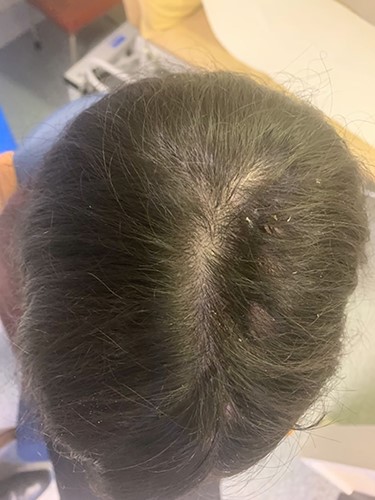
Final aesthetic result obtained 1 month after tissue expander removal.
DISCUSSION
Outlined below are 10 technical tips for the optimisation of tissue expanders:
Incisions: The authors advocate the use of small remote incisions for tissue expander access. Remote incisions permit the approximation of healthy tissue edges that may offer superior soft tissue healing. Some authors advocate incisions perpendicular to the axis of expansion to permit expedited filling and minimize the risk of dehiscence [4]; however, this approach is limited by the formation of another scar. Intralesional V- or W-shaped incision, which combine the advantages of both radial and tangential incisions and does not have their disadvantages, can be used.
Antibiotics: Provide local and systemic antibiotic prophylaxis and minimize the exposure time of the expander. The authors recommend a single dose of intravenous antibiotics effective against skin flora at induction combined with pocket irrigation using betadine triple-antibiotic solution to reduce biofilm formation [5].
Pocket dissection: The utilization of a lighted retractor facilitates dissection of an appropriately sized soft tissue pocket, see Fig. 2. The pocket should be larger than the expander and sited on a base that resists mechanical deformation to direct the force of expansion. In the illustrated case, the authors placed tissue expanders in the subgaleal plane to minimize stress on the overlying skin flap. The biomechanical properties of each tissue plane should be considered be when placing expanders. Tomita et al. [6] report significantly thinner capsules in subcutaneous versus subpectoral tissue expander placement, which may offer less postoperative shrinkage and greater predictability in soft tissue shape.
Multiple expanders: Consider the use of multiple tissue expanders, sited either side of the soft tissue defect. In anatomically suitable areas of high tissue laxity, the use of multiple expanders divides the volume of expansion required in a single area and reduces the overall duration of expansion. Such benefit must be balanced against increased risk of infection with multiple expanders [7].
Size: Select the largest tissue expander size able to fit within the soft tissue pocket. Radovan advised that the base of the tissue expander should be at least equal to the length of the defect to be covered to ensure adequate soft tissue expansion for resurfacing and tension free closure [8].
Shape: Rectangular expanders provide the greatest soft tissue surface-area gain. In vitro and in vivo models have demonstrated that rectangular tissue expanders provide a 38% increase in surface area compared with 25% in round expanders [9].
Port placement: When using an expander with an internally placed, remote valve port system, site the port away from the tissue expander to avoid unintentional perforation of the expander (see Fig. 2), but close enough to avoid a second incision. The authors advocate minimal undermining of the port pocket with the fixation of sutures if the risk for port migration is high. Port externalization may have a higher infection rate, although has the benefit of painless filling, which is pertinent in the paediatric population [7].
Drains: Place closed system suction drains within each tissue expander pocket to prevent haematoma and seroma formation as well as to minimize dead space. Drains should be sited adjacent to the flap pedicle and leading edge. Continuous infiltration of local anaesthetic has been shown to reduce acute pain and chronic pain as well as opioid consumption after tissue expander breast reconstruction, without significant risk of infection [10].
Layered closure: Close the skin in layers using an absorbable deep dermal suture and staples to reduce the chance of implant extrusion and exposure, see Fig. 3. A malleable retractor can be used to protect the tissue expander during closure of the deep dermal layer.
Timing and overexpansion: Begin soft tissue expansion 2 weeks after expander insertion. This lag permits sufficient time for wound healing, reducing the chance of wound dehiscence, where remote incisions are used. By 2 weeks, complications related to the skin flap typically manifest [1]. Expansion rate is dictated by the level of discomfort tolerated by the patient and clinical factors such as surface tension and the presence of cutaneous blanching. Expanders can be over-filled up to x2.5 their volume, although in the presented case, the patient could only tolerate x1.4 (140 ml) due to pain. Expedited, short interval filling may be possible with adjacent incisions for expander placement, multiple expanders and port externalization, although this must be tempered against the risk of implant externalization and infection.
Conflict of interest statement
The authors declare no conflict of interest related to the current work.
FUNDING
None.



