-
PDF
- Split View
-
Views
-
Cite
Cite
Selma Hetoja, Carlo Alberto De Pasqual, Valentina Mengardo, Jacopo Weindelmayer, Simone Giacopuzzi, Gastric conduit perforation after Ivor Lewis esophagectomy successfully treated with endoscopic vacuum therapy (E-VAC): a case report, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa251, https://doi.org/10.1093/jscr/rjaa251
Close - Share Icon Share
Abstract
Gastric conduit perforation is a life-threatening complication after esophagectomy and currently there is no consensus about its optimal management. Endoscopic vacuum therapy (E-VAC) is a promising technique for the treatment of leaks and perforations after upper gastro-intestinal surgery. We report the case of a 65 years-old male patient who underwent an Ivor Lewis esophagectomy for esophago-gastric junction adenocarcinoma. He referred to our Emergency Department for septic shock and right hydropneumothorax. We performed an emergency thoracoscopy with intraoperative esophagogastroduodenoscopy which showed a pre-pyloric perforation of the gastric conduit. The perforation was initially treated with unsuccessful primary surgical closure and subsequently by means of E-VAC, firstly placed intraluminal and then intracavitary. With the latter technique, we assisted to a progressive clinical improvement until the definitive healing of the perforation. To our knowledge, this is the first case of a gastric tube perforation after esophagectomy successfully treated with E-VAC.
INTRODUCTION
Esophagectomy is a mainstay in the management of esophageal and esophago-gastric junctional malignancies [1]. During esophagectomy, the digestive continuity is usually restored using the stomach tubularized on the greater curvature. Gastric tube ulcer is quite common, being reported in 2,6%-19,4% of cases [2]. One of its most dreaded complications is gastric tube perforation, which is associated with up to 84,6% mortality rates [3]. A tailored management is necessary, depending on the site of perforation and on patient conditions. Surgery is considered mandatory in case of rapidly developing sepsis, but this approach is associated with a high rate of mortality (9,5-13,8%) [4]. Endoscopic Vacuum Therapy (E-VAC) has been demonstrated to be feasible and safe in the management of anastomotic leaks and perforations after esophagectomy that otherwise would have required surgery, with a success rate of 66,7% to 100% [5].
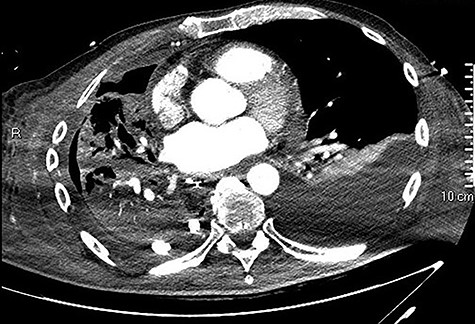
Initial CT showing a right hydropneumothorax after chest tube placement.
CASE REPORT
We present the clinical case of a 65 years old male patient submitted to totally minimally invasive Ivor Lewis esophagectomy after neoadjuvant chemo-radiotherapy for esophago-gastric junction adenocarcinoma (ypT2N0M0). A month after the surgery, the patient referred to our Emergency Department complaining acute dysphagia and asthenia. He presented with hypotension and tachycardia; laboratory blood tests revealed a
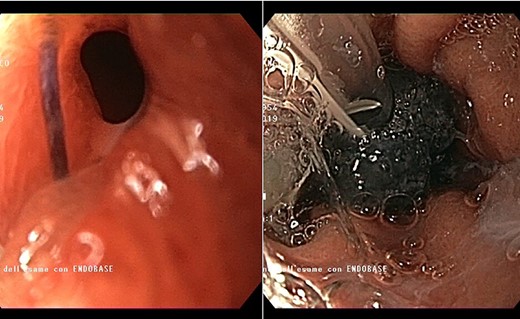
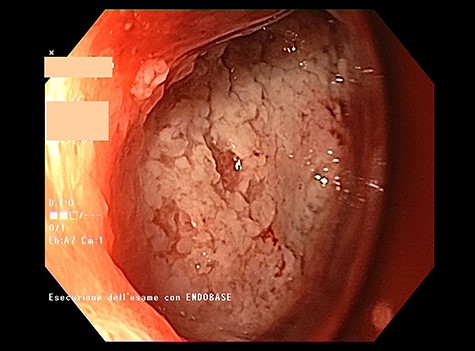
Endoscopic view of the prepyloric ulcer perforation (left) and intraluminal placement of E-VAC (right)
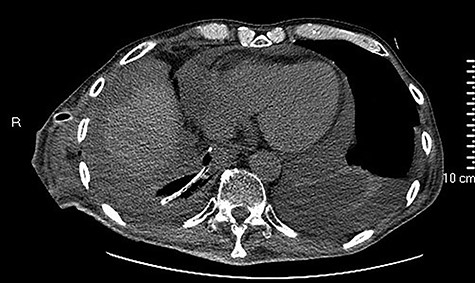
high PCR (291 mg/L) and the CT scan showed a right hydropneumothorax (Fig. 1). The first suspicion was an anastomotic leak and, in consideration of the hemodynamic instability and the severe septic state, the patient was submitted to emergency surgery. We performed a thoracoscopy with intraoperatively esophagogastroduodenoscopy which revealed a perforated prepyloric ulcer of the gastric tube. We therefore proceeded with accurate pleural toilette and closed the perforation with surgical stiches. At the end of the procedure, two thoracic drains and a naso-gastric tube with the distal end near the suture were placed. An additional naso-duodenum enteral feeding tube to ensure adequate nutrition was positioned. The patient was admitted in the Intensive Care Unit and supported with inotropic drugs, broad-spectrum antibiotics and proton pump inhibitor therapy. In the following days, the patient’s conditions improved allowing his extubation and his transfer to the surgical ward. Unfortunately, imaging and endoscopic check revealed a recurrent perforation. Considering the failure of the conservative approach, we decided to start the E-VAC Therapy on his 21st post-operative day (POD), using the Eso-SPONGE® System (B. Braun Surgical, S.A. Carretera De Terrassa, Rubi, Spain), firstly placed intraluminal because of the reduced size of the wall defect (Fig. 2). After three E-VAC replacements we did not observe any endoscopic or radiologic improvements. Moreover, on his 35th POD, due to a new impairment of vital signs, the patient underwent a second surgical treatment, with a new pleural toileting and another unsuccessful attempt of closing the conduit defect surgically. Considering the prolonged hospital stay, we placed a feeding jejunostomy. We proceeded with a new attempt of E-VAC, this time by placing it intracavitary (Fig. 3 and Fig. 4). The E-VAC was changed every 48–72 hours, reshaping the dimension of the sponge every time according to the size of the cavity. We observed a progressive reduction of the cavity size during the seriated endoscopic and CT evaluation. Considering the continuous improvement of patient’s clinical condition we discharged him after 84 days of hospitalization, with the E-VAC in place. The device replacement was carried on twice a week as an outpatient treatment, until its definitive removal (Fig. 5). The total duration of the therapy was 37 days with 13 E-VAC intracavitary interventions. Seven days after the device removal we performed an upper gastrointestinal X-Ray with oral contrast that showed no contrast medium leakage. The patient was therefore allowed to resume oral intake. At 6 months follow up the patient was in good clinical conditions; radiological test and endoscopy showed no abnormalities.
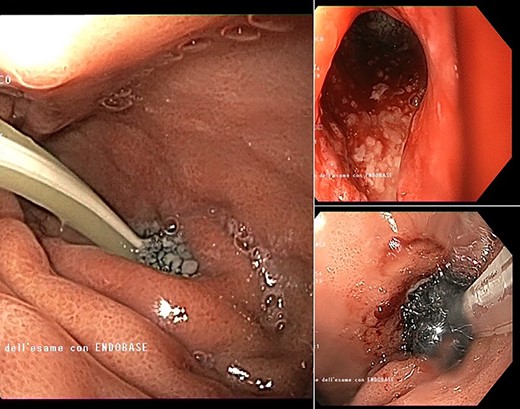
Intracavitary placement of E-VAC with prompt progressive improvement of the cavity size.
DISCUSSION
We report a case of gastric tube perforation after esophagectomy, refractory to traditional therapies and successfully treated with E-VAC. There are no guidelines on the management of gastric tube perforation and specific literature is scarce, with only few case reports published. In the clinical practice, the management of this complication is based on the same principles used for the treatment of anastomotic leak. The severity of patient’s clinical conditions should guide the management. Fluid resuscitation, antibiotic therapy and protonic pump inhibitors should be promptly started. Surgery should be considered in hemodynamically instable patients, in case of large defects or when conservative treatment has failed [6].
Interventional endoscopy has emerged as an effective and less invasive alternative to the surgical approach. E-VAC, especially, consists in a polyurethane sponge, endoscopically placed either in the lumen of the digestive system (intraluminal) or inside the wound cavity (intracavitary), which is mounted on a drain applying continuous negative pressure. Even though there is no consensus on its optimal indications, all patients with acute or chronic GI defects can be theoretically candidates to E-VAC [5]. Literature lacks randomized controlled trial comparing E-VAC to other treatments [7] and the current evidence derives from single-center retrospective studies. E-VAC, compared to other endoscopic approaches such as clips and stents, other than an apposition of wound edges provides also simultaneous internal drainage of purulent collections. After upper GI surgery, E-VAC has been shown to have a higher overall closure rate of anastomotic leaks in comparison to endoscopic self-expandable stent placement, 84,4% versus 53% respectively [8]. The choice of whether to place the sponge intraluminal or intracavitary is left to the single endoscopist, on the basis of the size of the wall defect, of the extraluminal cavity and personal experience. In our case, after the intracavitary placement we observed a prompt positive result since the first insertion. We did not observe adverse events associated to the intracavitary placement of the device, although cases of exsanguinating hemorrhage have been reported. Thus, although intraluminal placement might be safer and easier, the defect closure is harder to achieve with intraluminal placement alone [9]. A great disadvantage of E-VAC is the necessity for multiple endoscopic procedures, which requires a considerable commitment. A recent study, however, shows that the use of E-VAC for transmural upper GI defects is well tolerated by the patient, with a satisfactory long term quality of life [10]. To our knowledge this is the first report on the use of this device to treat the gastric conduit perforation after esophagectomy.
CONCLUSIONS
We reported a case of a gastric tube perforation after esophagectomy, an uncommon complication with high mortality rate, successfully treated with E-VAC therapy. E-VAC represents an innovative and feasible option for upper gastro-intestinal leaks. Further studies are needed to confirm these findings.
CONFLICT OF INTEREST STATEMENT
None declared.
References
- conduit implant
- septic shock
- adenocarcinoma
- upper gastrointestinal endoscopy
- endoscopy
- emergency service, hospital
- esophagectomy
- esophagogastric junction
- hydropneumothorax
- intestines
- intraoperative care
- surgical procedures, operative
- thoracoscopy
- pylorus
- surgery specialty
- esophagectomy, ivor lewis
- gastric tube reconstruction
- consensus
- surgical closure techniques



