-
PDF
- Split View
-
Views
-
Cite
Cite
Lara Witt, Yagan Pillay, Rathi M Sabaratnam, Richard J Bigsby, De novo adolescent gastric carcinoma: a first case report in Saskatchewan, Canada, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa249, https://doi.org/10.1093/jscr/rjaa249
Close - Share Icon Share
Abstract
Adolescent gastric cancers are extremely rare with a reported incidence of 0.05–0.10% in North America. We present a de novo case of gastric carcinoma in a 17-year-old teenager with no concomitant family history or risk factors. His main clinical presentation included anaemia and melaena stools. Despite an extensive clinical workup that included a diagnostic laparoscopy, the tumour was deemed surgically irresectable, and he was started on a palliative chemotherapy protocol at the local paediatric oncology centre. He demised 7 months later. This is the first recorded case of an adolescent gastric cancer in Saskatchewan, Canada. This case highlights the need for an international tumour registry to document and investigate rare adolescent gastric malignancies and thereby potentiate a possible cure through the pooling of limited resources.
INTRODUCTION
Gastric adenocarcinoma is an extremely rare subtype of adolescent malignancy, with the current literature describing an incidence in North America of 0.05–0.10% of all adolescent neoplasms [1–3]. Signet ring adenocarcinoma comprises 45% of adolescent gastric adenocarcinoma cases [3]. This documented rarity of gastric adenocarcinoma in the adolescent population results in a delayed diagnosis, with subsequent investigations invariably revealing an advanced pathological staging [3]. We describe a case of a signet ring adenocarcinoma in a 17-year-old male, the first documented case, to our knowledge, in Saskatchewan, Canada [4] (Tables 1 and 2).
The incidence of stomach cancer in Canadians aged 10–14 from 2010 to 2017 [4]. No data available for 2018–2020
| Incidence of stomach cancer in Canadians aged 10–14 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Incidence of stomach cancer in Canadians aged 10–14 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The incidence of stomach cancer in Canadians aged 10–14 from 2010 to 2017 [4]. No data available for 2018–2020
| Incidence of stomach cancer in Canadians aged 10–14 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Incidence of stomach cancer in Canadians aged 10–14 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The incidence of stomach cancer in Canadians aged 15–19 from 2010 to 2017 [4]. No data available for 2018–2020
| Incidence of stomach cancer in Canadians aged 15–19 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Incidence of stomach cancer in Canadians aged 15–19 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The incidence of stomach cancer in Canadians aged 15–19 from 2010 to 2017 [4]. No data available for 2018–2020
| Incidence of stomach cancer in Canadians aged 15–19 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Incidence of stomach cancer in Canadians aged 15–19 . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Region . | 2010 . | 2011 . | 2012 . | 2013 . | 2014 . | 2015 . | 2016 . | 2017 . |
| Newfoundland and Labrador | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Prince Edward Island | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nova Scotia | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| New Brunswick | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Quebec | 0 | .. | .. | .. | .. | .. | .. | .. |
| Ontario | 0 | 0 | 5 | 0 | 5 | 0 | 0 | 0 |
| Manitoba | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 |
| Saskatchewan | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Alberta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| British Columbia | 5 | 0 | 0 | 0 | 5 | 0 | 0 | 0 |
| Yukon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Northwest Territories | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nunavut | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
CASE REPORT
A 17-year-old Indigenous male presented to our emergency department with a complaint of melaena stools for the past 3 months. He denied a history of haematemesis, haematochezia and previous surgeries and had no known allergies. His past medical history comprised of one previous emergency room visit a month earlier at symptom onset which required a blood transfusion for a haemoglobin level of 7.0 mg/dl. Blood parameters at his initial presentation demonstrated iron deficiency anaemia, although the aetiology for the anaemia was never ascertained. His family history was unremarkable with no history of gastric cancers and gastrointestinal malignancies or personal history of malignancy.
Clinically, the patient was emaciated, with a scaphoid abdomen, and extremely pale. He did not appear jaundiced, and a digital rectal exam yielded fresh melaena stools. Physical examination disclosed no evidence of palpable lymph nodes or abdominal masses. His haemoglobin level was 7.5 mg/dl and he was transfused two units of packed cells. The complete blood count showed an iron deficiency anaemia once again, and his expanded biochemistry profile was normal.
Urgent gastroscopy revealed a giant gastric ulcer in the antrum (Fig. 1) which was biopsied and sent for pathological investigation. The ulcer displayed no bleeding stigmata. He was started on eradication therapy for Helicobacter pylori. Pathology revealed a signet ring adenocarcinoma of the stomach (Fig. 2A and B). A computerized tomography (CT) scan (Fig. 3) showed a large mass in the distal stomach, and the ensuing positron emission tomography (PET) scan (Fig. 4) showed enlarged D1 perigastric lymph nodes with no obvious metastatic disease.
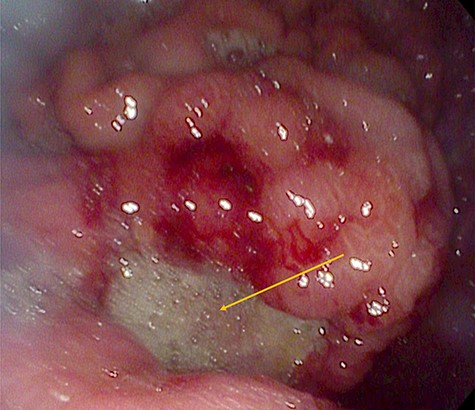
A giant gastric ulcer in the antrum during gastroscopy (orange arrow).
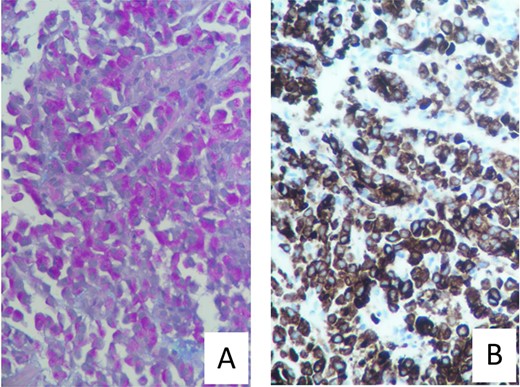
(A) Mucicarmine stain with diffuse sheets of malignant cells and rare glandular formation. The mucin is intracytoplasmic. (B) Pankeratin immunohistochemistry with positive Pankeratin confirms the epithelial linage of the malignant infiltration.
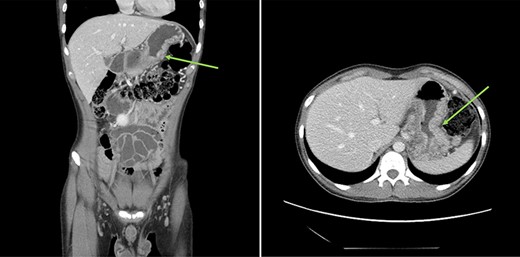
: (A and B) CT scans (coronal and axial views) showing a large mass in the distal stomach (green arrows).
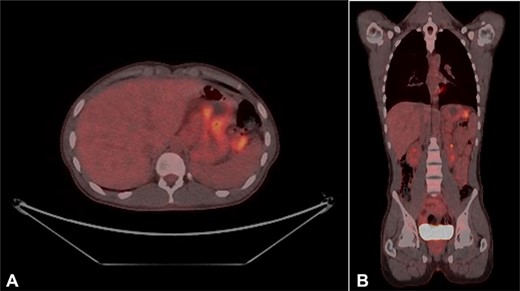
(A and B) PET scans (axial and coronal views) showing enlarged D1 perigastric lymph nodes (green arrows) and no apparent metastatic disease.
At diagnostic laparoscopy, the tumour was deemed surgically irresectable with large clinically visible perigastric lymph nodes (Fig. 5). The patient was referred to surgical oncology for neoadjuvant chemotherapy on a palliative protocol. The patient succumbed 7 months later in the hospital.
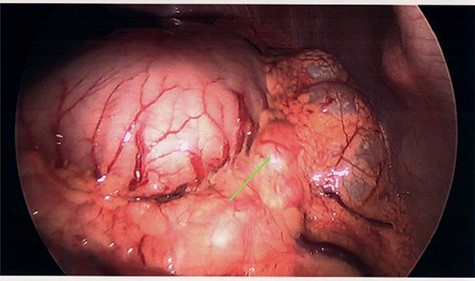
Clinically irresectable gastric tumour with large, visible perigastric lymph nodes, visualized on diagnostic laparoscopy (green arrow).
DISCUSSION
Adolescent gastric cancers are a rare affliction, with only 25 cases recorded in Canada since 2010 [4] (Tables 1 and 2). No previous cases have been described in Saskatchewan, although the provinces of Ontario, British Columbia and Manitoba have all recorded cases during this period [4]. In the adult demographic, risk factors such as H. pylori infection, atrophic gastritis, smoking, male sex, advanced age and positive family history are associated with a higher risk of gastric adenocarcinoma development [5]. Traditional risk factors are unreliable diagnostic indicators in the adolescent population as gastric adenocarcinomas manifest as de novo occurrences expressed via genetic mutations or develop as a side effect of gastric lymphoma treatment [1, 6, 7]. Recent literature suggests that adolescents with de novo gastric carcinomas behave oncologically different than their adult counterparts. This is demonstrated by a 2-fold increase in SRA risk, a significantly lower proportion of primary tumours in the gastric cardia, advanced staging at diagnosis, frequent nodal involvement and poorly differentiated histology [3, 8].
The presenting symptoms of adolescent gastric cancers are typically vague or flu-like on initial presentation. Symptoms include abdominal pain, anorexia, haematemesis, melaena, haematochezia, variable mass effect symptoms and a palpable abdominal mass [2, 6, 9]. Treatment of adolescent gastric adenocarcinoma includes prompt surgical resection of operable tumours with concurrent chemotherapy and radiation [3]. Adolescents typically present with aggressive, advanced tumours but are usually healthy and able to withstand more invasive interventions than their adult counterparts with indolent disease, resulting in a comparable survival rate between the two population groups. Regardless of age, gastric adenocarcinoma is associated with a poor prognosis, and the median survival in North America is 5 months from the initial diagnosis [10].
The poor prognosis associated with a diagnosis of gastric adenocarcinoma necessitates early gastroscopy and biopsy in adolescent patients presenting with concerning gastrointestinal symptoms to expedite diagnosis and treatment. This must be accompanied by a sharp clinical acumen, as these symptoms may initially appear benign. The sinister onset of symptoms and poor prognosis in adolescents suggest that clinical signs may be an augury of a rapidly growing, irresectable, advanced stage entity. This was certainly the case with our patient.
To prevent such senseless loss in the future, clinicians require considerably more than apt clinical judgement and a functional CT scanner. An international tumour registry for rare paediatric and adolescent cancers is an essential first step in the race to overcome these unusual and aggressive malignancies.
Resected specimens should be sent for genetic screening and cataloguing which can help ascertain risk factors that predispose certain adolescents to developing uncommon cancers [1, 10]. Identifying these adolescent, at-risk patients early is important to provide them and their families with all possible treatment options from the onset of disease. This would help reduce the disease burden on this population cohort and possibly contribute to a potential cure in the near future (1, 10). This pooling of medical resources will stand us in good stead given the extremely low incidence of adolescent malignancies which precludes the ability for one group of physicians to study these tumours closely.
CONCLUSION
We present the first recorded case of an adolescent gastric cancer in Saskatchewan, Canada, and strongly advocate for the formation of an adolescent tumour registry.
CONFLICT OF INTEREST STATEMENT
None declared.
FUNDING
None.
References
Author notes
Contributing authors.
Primary author.



