-
PDF
- Split View
-
Views
-
Cite
Cite
Paul Ghaly, Jim Iliopoulos, Mehtab Ahmad, Acute bilateral renal vein thrombosis diagnosis and management: a case report, Journal of Surgical Case Reports, Volume 2020, Issue 8, August 2020, rjaa238, https://doi.org/10.1093/jscr/rjaa238
Close - Share Icon Share
Abstract
Undifferentiated abdominal pain accounts for a significant proportion of emergency presentations and often presents as a diagnostic dilemma. Renal vein thrombosis (RVT) has many aetiologies including nephrotic syndrome, malignancy, trauma, infection and hypercoagulable states. RVT should be considered in cases of persistent abdominal pain where other, more common, pathologies have been excluded. We present the case of a 42-year-old male with a delayed diagnosis of bilateral RVT after presenting with multiple episodes of intractable abdominal pain and adverse sequelae of this condition. This case report aims to emphasize the importance of prompt RVT recognition and the utility of bedside emergency department (ED) investigations, which can guide initial differential diagnoses of abdominal pain, reduce the delay in diagnosis as well as limit unnecessary investigations.
INTRODUCTION
Bilateral renal vein thrombosis (RVT) is a rare occurrence. Prompt diagnosis of RVT can be challenging as symptoms are dependent on the rate of thrombus formation and extent of vessel occlusion. RVT signs and symptoms vary from flank pain, haematuria, nausea and vomiting—to a rapid decline in renal function and associated complications. However, most commonly RVT is asymptomatic [1–3]. The overlap in symptoms with pathologies such as renal colic often results in misdiagnosis. Most commonly, RVT is a result of hypercoagulable states such as nephrotic syndrome, as first described in the 1840s by the French Nephrologist, Rayer. [3] Other common systemic and local causes of RVT include primary hypercoagulable disorders, malignant renal tumours, trauma and infection [1–3]. Hence, the possibility of RVT needs to be considered with presentations of undifferentiated, intractable flank pain. Furthermore, its complexity highlights the utility of accessible, cost-effective bedside diagnostic tools in alerting physicians early to the diagnosis.
CASE REPORT
A 42-year-old male with a history of hyperlipidaemia self-presented multiple times to the emergency department (ED) with undifferentiated flank pain. On initial presentation, he complained of a 3-week history of diffuse abdominal pain radiating to the flanks, which was exacerbated on deep inspiration. He gave no history of recent long-haul travel or prolonged periods of immobility. He had no known allergies, was a life-long non-smoker with no family history of malignancy or hypercoagulable disorders. He was not on any medications for his hyperlipidaemia.
On examination in ED, his haemodynamic parameters were all within normal limits—heart rate 90 beats/min, blood pressure 131/94 mmHg, respiratory rate 20 breaths/min, saturations 96% on room air and temperature 37.1°C. He had bilateral upper quadrant abdominal tenderness on deep palpation. His cardiorespiratory examination was normal, with no weight loss, lower limb oedema or palpable lymphadenopathy.
Chest radiograph demonstrated signs of left basal atelectasis, subsequently leading to the diagnosis of community-acquired pneumonia and discharge on oral antibiotics. Given the abdominal pain, other differential diagnoses included pulmonary embolism (PE) and renal colic. PE was excluded at initial presentation using the Pulmonary Embolism Rule-out Criteria (PERC) [4]. Blood work showed a leucocytosis and neutrophilia (Table 1). A computerized tomography urogram (CTU) obtained for suspicion of renal colic showed no renal tract obstruction, subtle right peri-nephric fat stranding and left lower lobe consolidation (Fig. 1).
| Na . | 139 . | mmol/L . |
|---|---|---|
| K | 3.9 | mmol/L |
| Cl | 104 | mmol/L |
| Creatinine | 71 | μmol/L |
| Bilirubin | 9 | μmol/L |
| Albumin | 34 | g/L |
| ALP | 73 | U/L |
| GGT | 59 | U/L |
| ALT | 27 | U/L |
| AST | 21 | U/L |
| Amylase | 81 | U/L |
| Lipase | 39 | U/L |
| WCC | 16.9 | x10^9/L |
| Neutrophils | 13.5 | x10^9/L |
| Hb | 153 | 141 g/L |
| Platelets | 153 | x10^9/L |
| Troponin T (Highly sensitive) | 6 | ng/L |
| Urine culture | Nil organisms grown |
| Na . | 139 . | mmol/L . |
|---|---|---|
| K | 3.9 | mmol/L |
| Cl | 104 | mmol/L |
| Creatinine | 71 | μmol/L |
| Bilirubin | 9 | μmol/L |
| Albumin | 34 | g/L |
| ALP | 73 | U/L |
| GGT | 59 | U/L |
| ALT | 27 | U/L |
| AST | 21 | U/L |
| Amylase | 81 | U/L |
| Lipase | 39 | U/L |
| WCC | 16.9 | x10^9/L |
| Neutrophils | 13.5 | x10^9/L |
| Hb | 153 | 141 g/L |
| Platelets | 153 | x10^9/L |
| Troponin T (Highly sensitive) | 6 | ng/L |
| Urine culture | Nil organisms grown |
| Na . | 139 . | mmol/L . |
|---|---|---|
| K | 3.9 | mmol/L |
| Cl | 104 | mmol/L |
| Creatinine | 71 | μmol/L |
| Bilirubin | 9 | μmol/L |
| Albumin | 34 | g/L |
| ALP | 73 | U/L |
| GGT | 59 | U/L |
| ALT | 27 | U/L |
| AST | 21 | U/L |
| Amylase | 81 | U/L |
| Lipase | 39 | U/L |
| WCC | 16.9 | x10^9/L |
| Neutrophils | 13.5 | x10^9/L |
| Hb | 153 | 141 g/L |
| Platelets | 153 | x10^9/L |
| Troponin T (Highly sensitive) | 6 | ng/L |
| Urine culture | Nil organisms grown |
| Na . | 139 . | mmol/L . |
|---|---|---|
| K | 3.9 | mmol/L |
| Cl | 104 | mmol/L |
| Creatinine | 71 | μmol/L |
| Bilirubin | 9 | μmol/L |
| Albumin | 34 | g/L |
| ALP | 73 | U/L |
| GGT | 59 | U/L |
| ALT | 27 | U/L |
| AST | 21 | U/L |
| Amylase | 81 | U/L |
| Lipase | 39 | U/L |
| WCC | 16.9 | x10^9/L |
| Neutrophils | 13.5 | x10^9/L |
| Hb | 153 | 141 g/L |
| Platelets | 153 | x10^9/L |
| Troponin T (Highly sensitive) | 6 | ng/L |
| Urine culture | Nil organisms grown |
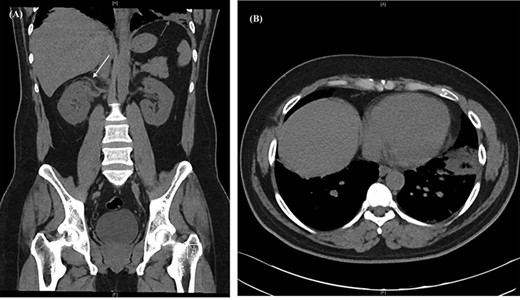
CT urogram showing (A) right peri-nephric fat stranding (white arrow) with left lower lobe consolidation (red arrow) and (B) transverse section highlighting the left basal consolidation.
Ongoing abdominal and chest pain prompted re-presentation to the ED within the same week. A computerized tomography pulmonary angiogram (CTPA) revealed bilateral segmental pulmonary emboli. His examination and blood panel remained unchanged from the initial presentation. Within the context of an unprovoked PE, lower limb Doppler studies and a thrombophilia screen were performed, which returned negative. Oral anticoagulation was commenced in the form of Apixaban, and he was discharged from the ED with a scheduled follow-up with his family physician.
Post-discharge, he continued to experience worsening abdominal pain prompting further re-presentation. An abdominal CT with contrast showed bilateral RVT with infra-hepatic extension into the inferior vena cava (IVC) (Fig. 2). The patient was admitted under the vascular unit for a comprehensive workup.
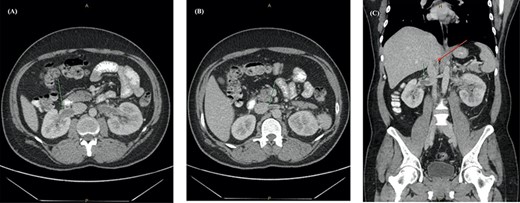
Abdominal CT with contrast showing (A) an enlarged right renal vein with a filling defect, (B) left renal vein distension and filling defect with (C) extension into the lower intra-hepatic inferior vena cava (red arrow).
Bedside urine analysis performed on admission revealed heavy proteinuria, which was further quantified by 24-hour urine protein studies (4 g protein/day). A pro-thrombotic screen including lupus anticoagulant, factor V Leiden, protein C and S and prothrombin genotyping were all negative. An autoimmune profile was strongly positive for anti-PLA2R antibodies. Antinuclear antibody test and extractable nuclear antigens tests were negative. The patient was commenced on subcutaneous enoxaparin 90 mg per day and perindopril for proteinuria. Given the clinical findings and strongly positive anti-PLA2R antibody, diagnosis of primary membranous nephropathy was made by the nephrologist, with the decision to forgo a renal biopsy due to bleeding risk. He was discharged with a planned nephrology review for future immunosuppressive therapy.
DISCUSSION
RVT refers to thrombus formation in the main trunk, or branches of the renal veins. It most commonly occurs unilaterally, whilst bilateral RVT is a rare complication of nephrotic syndrome. Nephrotic syndrome predisposes patients to developing thromboembolisms, particularly RVT. Membranous glomerulonephritis, minimal change disease and membranoproliferative glomerulonephritis, respectively, are the most commonly associated nephropathies [1, 3].
The pathogenesis of RVT involves an interplay of Virchow’s triad, namely, endothelial damage, stasis and hypercoagulability, and is often precipitated by a combination of two or more of these factors.
Clinical presentation varies with time of onset and degree of occlusion. Acute RVT may present with symptoms similar to renal colic such as flank pain, rapid decline in renal function, microscopic or macroscopic haematuria, nausea, vomiting and fevers. Chronic RVT is often asymptomatic.
Renal venography remains the gold standard imaging modality; however this has been superseded by less invasive diagnostic methods. CT angiography is the imaging of choice, with almost 100% sensitivity and specificity. Contrast-enhanced magnetic resonance venography is of equivalent sensitivity, with the added benefit of radiation avoidance [3, 5].
Given the strong association with nephrotic syndrome, bedside urine dipstick screening for proteinuria is a cost-effective tool for the prompt recognition of possible nephrotic syndrome in the undifferentiated abdominal pain patient, with 96% sensitivity for detecting heavy proteinuria (≥3 + protein) [6, 7].
Management of RVT involves treating the underlying precipitant, protecting renal function and prevention of complications. Anticoagulation is recommended to prevent thrombus progression and emboli. Unfractionated heparin or low-molecular-weight heparin are typically initiated, with bridging to warfarin for 6–12 months, or until resolution of the underlying nephrotic disease. In acute RVT, thrombectomy and/or thrombolysis indications include bilateral RVT, treatment failure whilst on systemic anticoagulation, thrombosis of a solitary or transplanted kidney and thrombus extension into the IVC. Fibrinolysis is associated with significant improvement in renal function and in the absence of contraindications, a low risk of bleeding [2, 8].
RVT is a rare complication of hypercoagulable states, which often mimics presentations of common abdominal pathologies. Its occurrence should be highly suspected upon the exclusion of pathologies such as urolithiasis and renal colic. As seen in our case, early utilization of easily accessible bedside investigations could potentially aid the prompt diagnosis and management of RVT, thereby avoiding unnecessary, invasive investigations and their associated complications.



